A kind of pure organic room temperature phosphorescent polymer material with lactam ring structure and its preparation method
A technology of polymer materials and lactam rings, applied in the field of pure organic room temperature phosphorescent polymer materials and their preparation, to achieve the effect of good phosphorescence characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A preparation method of a pure organic room-temperature phosphorescent polymer material with a lactam ring structure, the specific steps are as follows: Weigh 33mg of benzoic acid compounds, 10mg of azobisisobutyronitrile and dissolve them in 1.5mL of ethanol, add 1.5mL of N-vinylpyrrolidone , dissolved, blown air with a needle, and reacted for 12 hours at 70°C under the protection of nitrogen. The obtained product was dropped into acetone for beating, the clear liquid was removed, and the pure organic room temperature phosphorescent polymer material was obtained after rotary evaporation and drying.
[0029] The specific structure of the obtained product polymer is as follows:
[0030]
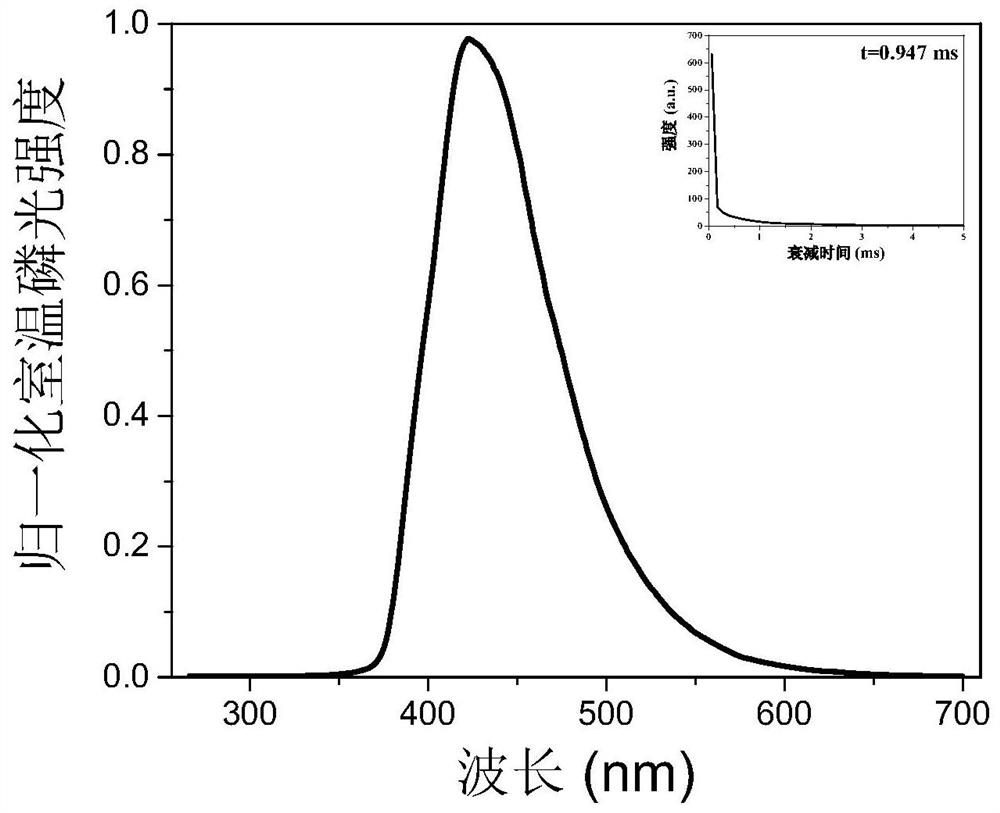
[0031] Its phosphorescent emission peak is around 428nm (see figure 1 ), the lifetime is 0.947ms, and the quantum yield is 0.8%.
Embodiment 2
[0033] A preparation method of a pure organic room-temperature phosphorescent polymer material with a lactam ring structure, the specific steps are as follows: Weigh 38 mg of bronaphthalimide compounds, 8 mg of azobisisobutyronitrile and dissolve them in 1 mL of N,N-dimethylformaldehyde Add 1mL of N-vinylpyrrolidone to the amide, dissolve it, inflate it with a needle, and react at 70°C for 12h under the protection of nitrogen. The obtained product was dropped into acetone for beating, the supernatant liquid was removed, and the pure organic room-temperature phosphorescent polymer material was obtained after rotary evaporation and drying.
[0034]
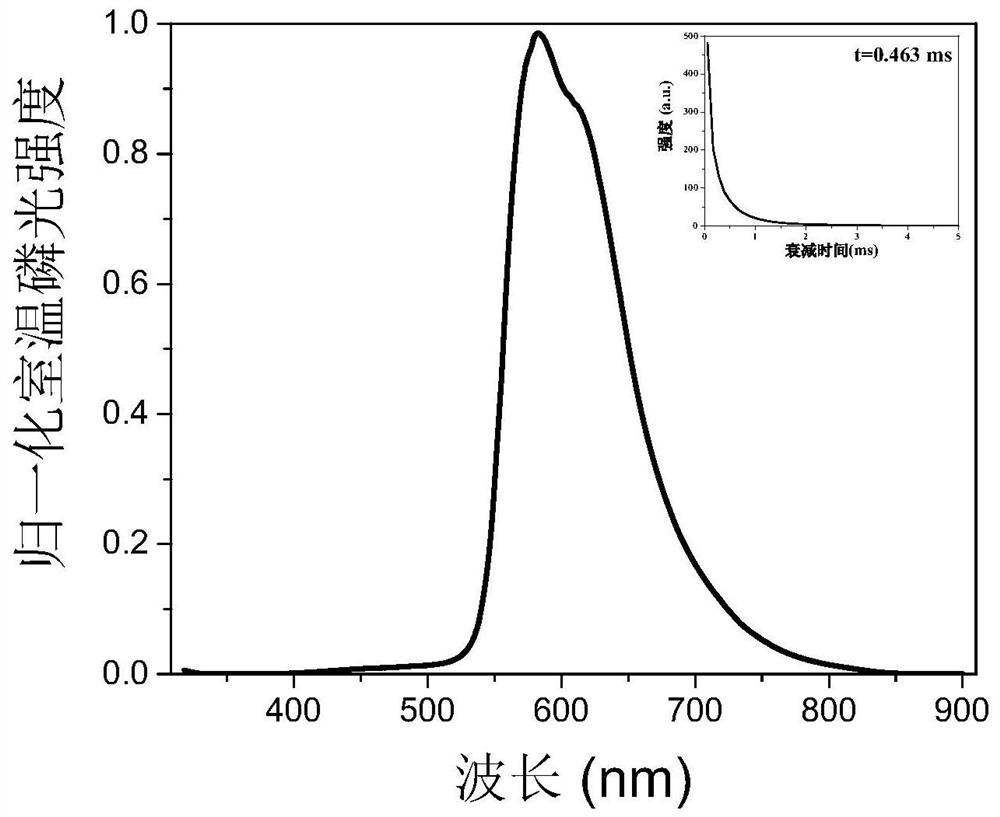
[0035] Its phosphorescent emission peak is around 582nm (see figure 2 ), and has a companion peak around 610nm, a lifetime of 0.463ms, and a quantum yield of 1.6%.
Embodiment 3
[0037] A method for preparing a pure organic room-temperature phosphorescent polymer material with a lactam ring structure, the specific steps are as follows: Weigh 20 mg of azobisisobutyronitrile and dissolve it in 2 mL of ethanol, add 2 mL of N-vinylpyrrolidone, dissolve, and blow air through a needle. After air blowing, inject 204mg of 6-bromo-1-hexene monomer with a needle, and react at 70°C for 12h under the protection of nitrogen. The obtained product was dropped into diethyl ether for beating, the clear liquid was removed, and the pure organic room-temperature phosphorescent polymer material was obtained after rotary evaporation and drying.
[0038] The specific structure of the obtained product polymer is as follows:
[0039]
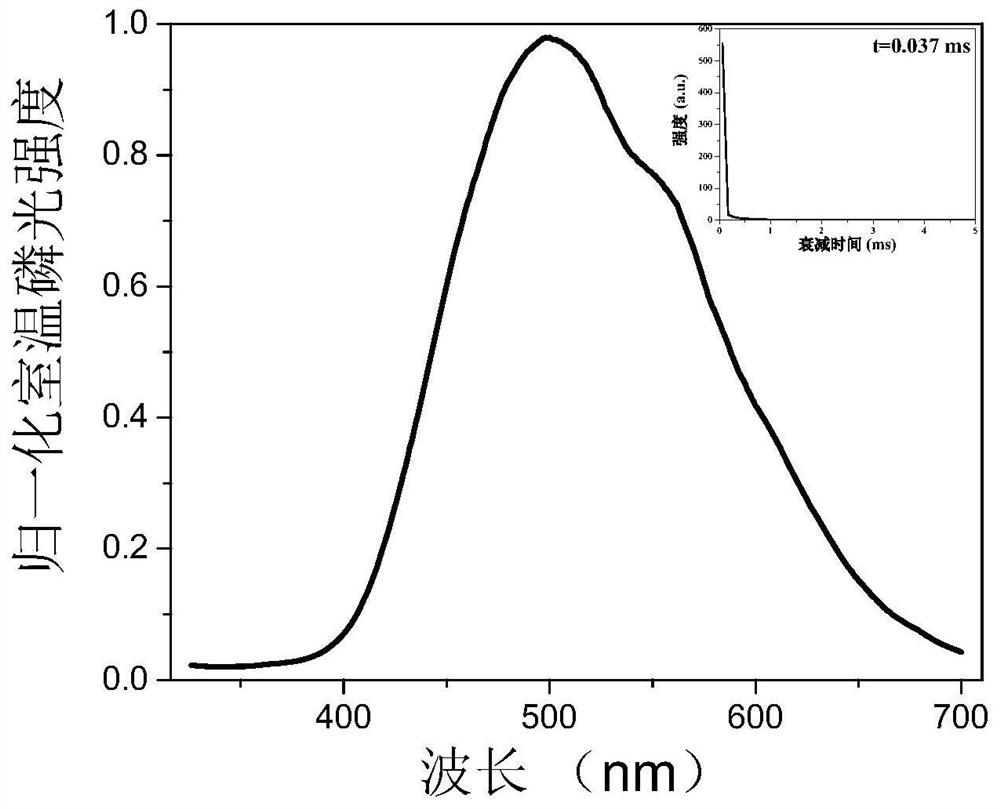
[0040] Its phosphorescent emission peak is around 496nm (see image 3 ), the lifetime is 0.037ms, and the quantum yield is 0.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com