Near-infrared fluorescent dye and preparation method thereof
A fluorescent dye and near-infrared technology, applied in the direction of oxazine dyes, organic dyes, luminescent materials, etc., can solve the problems of restricting the application of dyes, difficult to modify, easy to aggregate, etc., and achieve good application prospects, high profits, and good solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
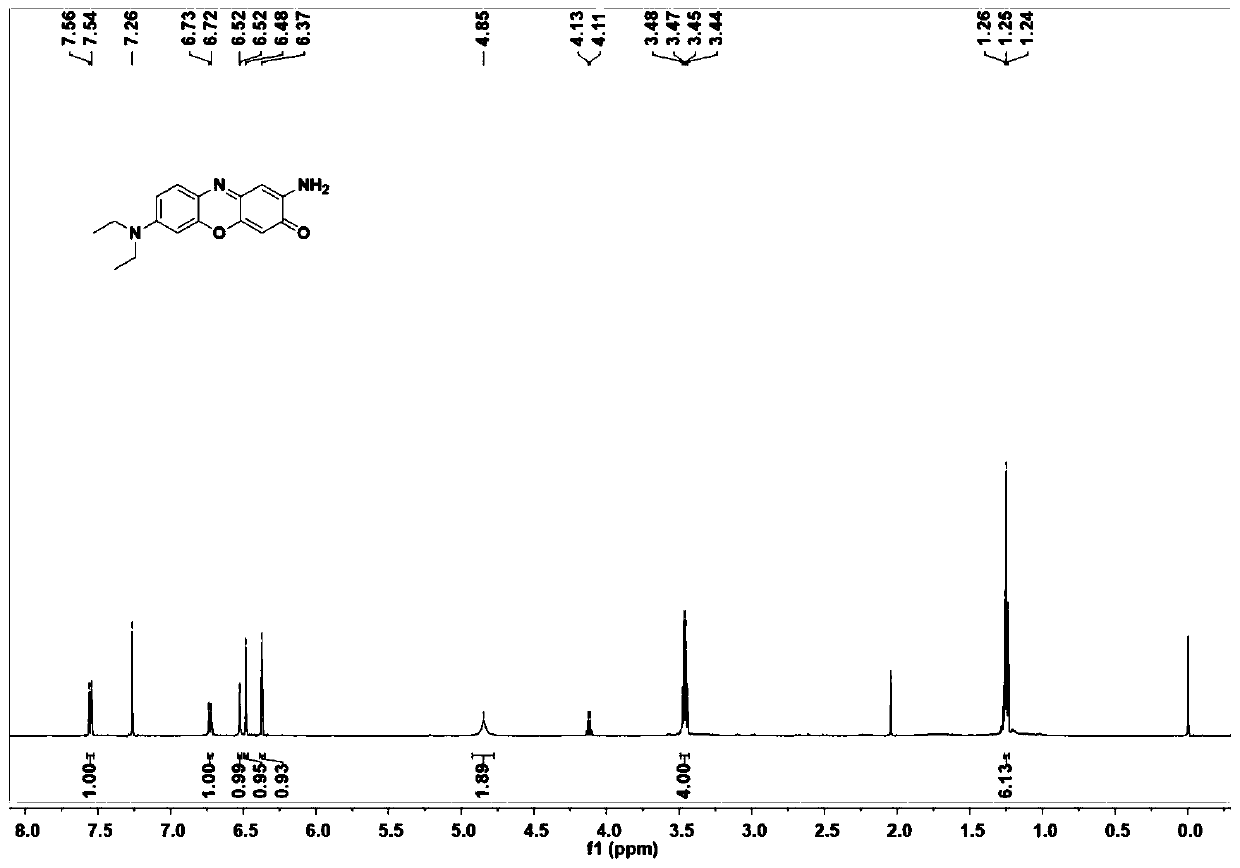
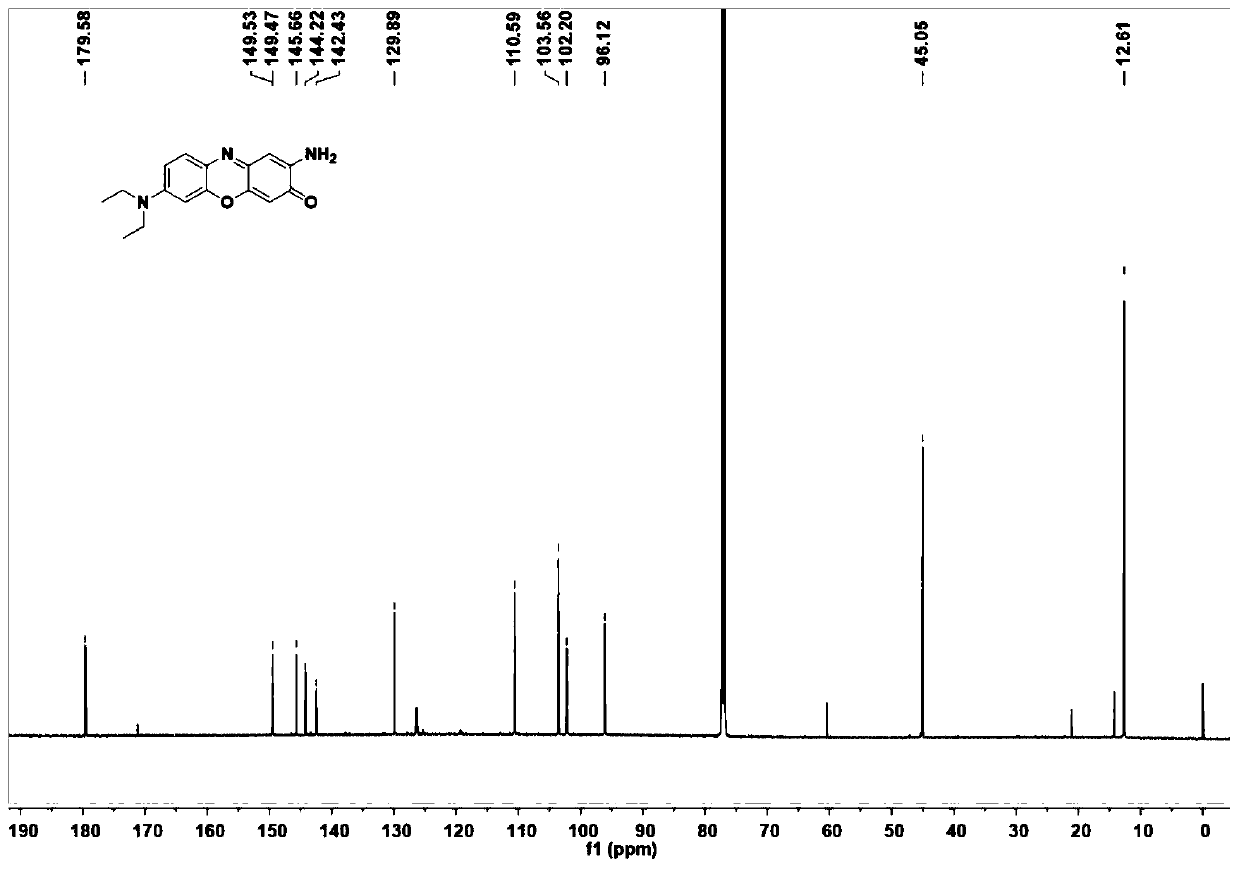
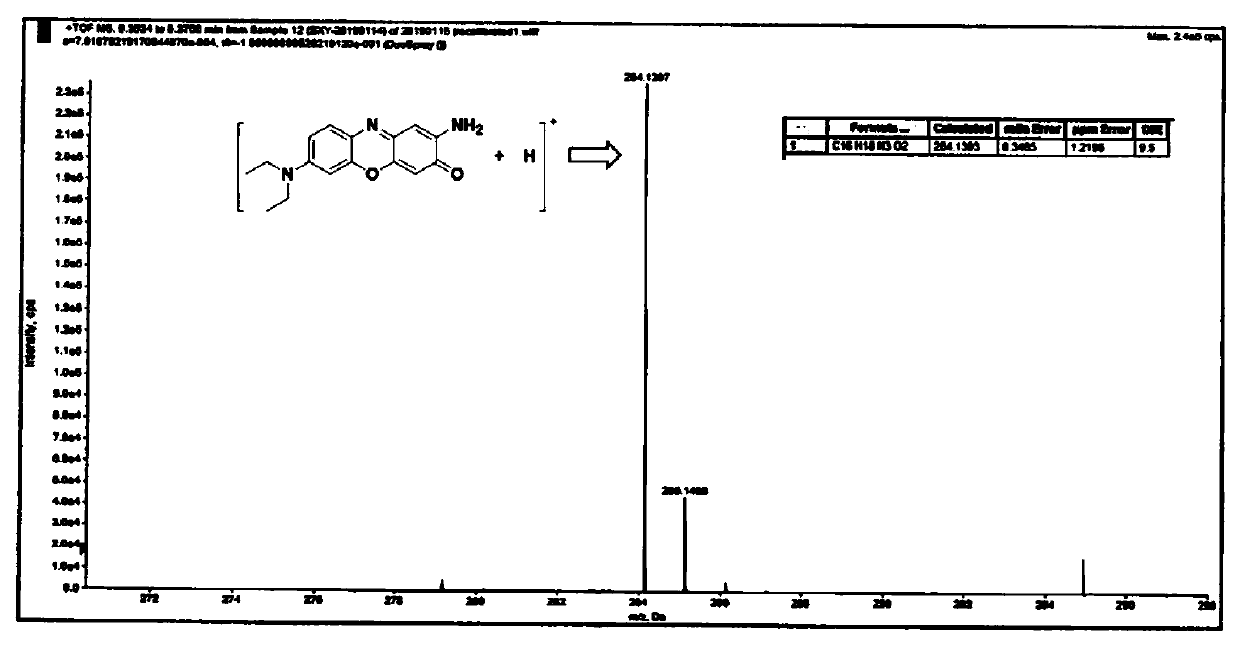
[0033] This embodiment provides a kind of near-infrared fluorescent dye, and its structural formula is as shown in (a):
[0034]
[0035] The synthetic route of described near-infrared fluorescent dye is shown in (b):
[0036]
[0037] The preparation method of described near-infrared fluorescent dye comprises the following steps:
[0038] (1) Xiangcong H 2 SO 4 Add N,N-diethyl-3-hydroxyaniline, stir to dissolve the raw material, add NaNO 3 , continue to stir until the reaction is complete, pour the reaction solution into ice water, a large amount of brownish-yellow solid is precipitated, filter under reduced pressure, wash the filter cake with deionized water, and dry in vacuum to obtain the filter cake compound;
[0039] (2) Dissolve the filter cake compound obtained in step (1) in ethanol, react in a hydrogen atmosphere, remove the hydrogen when the color of the reaction solution becomes colorless, allow air to enter the reaction bottle, and the color of the soluti...
Embodiment 2
[0041] This embodiment provides a kind of near-infrared fluorescent dye, and its structural formula is as shown in (a):
[0042]
[0043] The synthetic route of described near-infrared fluorescent dye is shown in (b):
[0044]
[0045] The preparation method of described near-infrared fluorescent dye comprises the following steps:
[0046] (1) In an ice bath, add 40 mL of concentrated H 2 SO 4 Add 30mmol of N,N-diethyl-3-hydroxyaniline, stir to dissolve the raw material, keep ice bath and add 33mmol of NaNO 3 , continue to stir at room temperature for 7 hours. After the reaction is completed, slowly pour the reaction solution into 200mL of ice water, and a large amount of brown-yellow solid is precipitated. After vacuum filtration, the filter cake is washed repeatedly with deionized water for at least three times, and vacuum-dried to obtain the filter cake compound ;
[0047](2) Dissolving the filter cake compound in ethanol, adding palladium carbon as a catalyst, re...
Embodiment 3
[0049] This embodiment provides a kind of near-infrared fluorescent dye, and its structural formula is as shown in (a):
[0050]
[0051] The synthetic route of described near-infrared fluorescent dye is shown in (b):
[0052]
[0053] The preparation method of described near-infrared fluorescent dye comprises the following steps:
[0054] (1) Under ice-bath conditions, in the presence of concentrated H 2 SO 4 (40mL) into a 100mL round bottom flask, add N,N-diethyl-3-hydroxyaniline (4.9573g, 30mmol), stir vigorously to dissolve the raw material, keep the ice bath and slowly add NaNO 3 (2.805g, 33mmol), stirred at room temperature for 7h, after the reaction was completed, the reaction solution was slowly poured into 200mL of ice water, a large amount of brown-yellow solid was precipitated, filtered under reduced pressure, and the filter cake was washed repeatedly with deionized water (50mL*3), Vacuum dry.
[0055] (2) The obtained filter cake compound is dissolved in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com