2-aminopyrimidine compound and application thereof
An aminopyrimidine and compound technology, which can be used in compounds, drug combinations, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
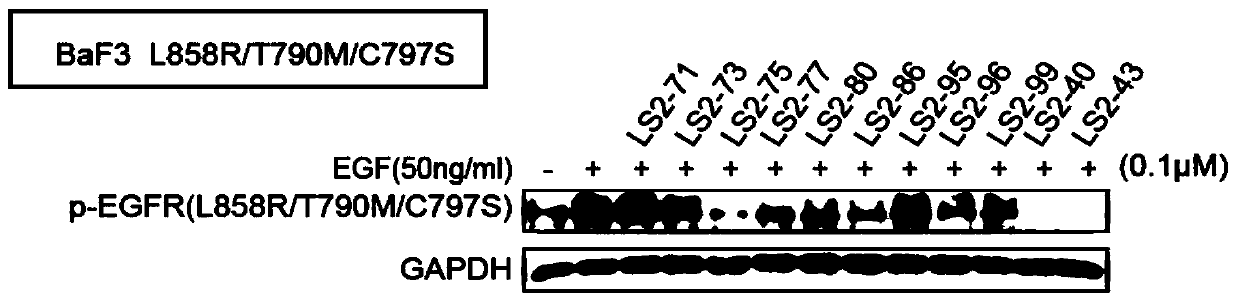
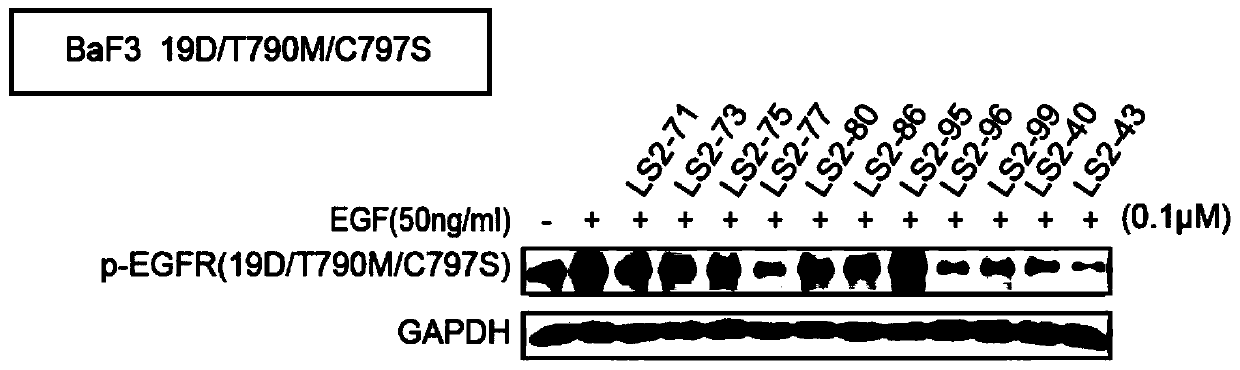
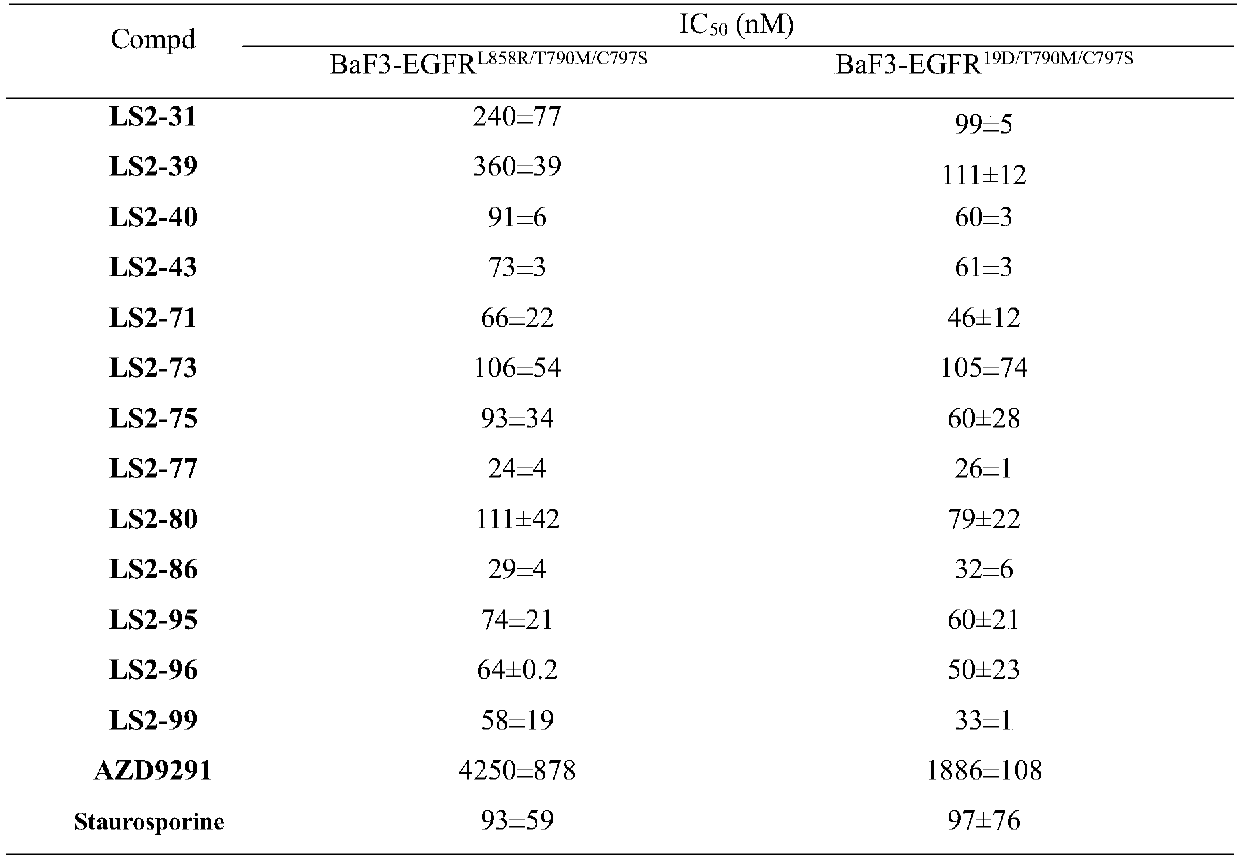
Image
Examples
Embodiment 1
[0166] (2-((5-chloro-2-((4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)-3-trifluoromethylphenyl)amino)pyrimidine -4-yl) amino) phenyl) dimethyl phosphorus oxide (LS2-31)
[0167] The synthetic route is as follows:
[0168]
[0169] Step 1. Preparation of (2-aminophenyl) dimethyl phosphorus oxide (2)
[0170] O-iodoaniline (1,5.06g, 23mmol), dimethyl phosphorus oxide (2.20g, 27.2mmol), palladium acetate (0.26g, 1.2 mmol), 4,5-bisdiphenylphosphine-9,9- Dimethylxanthene (0.67g, 1.2mmol) and potassium phosphate (5.40g, 25.4mmol) were dissolved in 50ml of N,N-dimethylformamide solvent, and reacted overnight at 120°C under argon protection. After the reaction was complete, most of the solvent was spin-dried under reduced pressure, extracted three times with dichloromethane / water, the organic layers were combined, washed with saturated brine, dried with anhydrous sodium sulfate and spin-dried, and separated by column chromatography to obtain a solid 3.2 g, yield 82%.
[0171] 1...
Embodiment 2
[0190] (2-((5-chloro-2-((5-methyl-6-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)pyridin-3-yl)amino) Pyrimidin-4-yl)amino)phenyl)dimethylphosphorus oxide (LS2-39)
[0191]
[0192] The synthetic method refers to Example 1, and the yield is 67%.
[0193] 1 H NMR (400MHz, CDCl 3 )δ8.45-8.35(m,1H),8.18(d,J=2.5Hz,1H),8.08(s,1H),7.84 (d,J=2.5Hz,1H),7.62(ddd,J 1 =14.1Hz,J 2 =7.7Hz,J 3 =1.4Hz, 1H), 7.54(t, J=7.9Hz, 1H), 7.32-7.20(m, 1H), 3.39(d, J=12.4Hz, 2H), 3.09-2.65(m, 9H), 2.64 -2.32(m,5H),2.22(s,3H), 2.08-1.93(m,2H),1.87(s,3H),1.83(s,3H),1.76-1.65(m,2H).
[0194] MS(ESI):m / z 569[M+H] + .
Embodiment 3
[0196] (2-((5-chloro-2-((3-chloro-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)phenyl)amino)pyrimidine-4- Base) amino) phenyl) dimethyl phosphorus oxide (LS2-40)
[0197]
[0198] The synthetic method refers to Example 1, and the yield is 58%.
[0199] 1 H NMR (400MHz, CDCl 3 )δ10.94(s,1H),8.58(dd,J 1 =8.4Hz,J 2=4.4Hz, 1H), 8.08(s, 1H), 7.76(d, J=2.5Hz, 1H), 7.55(t, J=7.9Hz, 1H), 7.33-7.28(m, 1H), 7.21(dd ,J 1 =8.6Hz,J 2 = 2.5Hz,1H),7.16-7.08(m,1H),6.98(d,J=8.7Hz,1H),6.84(s,1H),3.40(d,J=11.7Hz,2H), 2.84-2.46 (m,9H),2.46-2.37(s,1H),2.33(s,3H),2.05-1.89(m,3H),1.84(d,J=13.1Hz,6H), 1.81-1.73(m,2H ).
[0200] MS(ESI):m / z 588[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com