Telmisartan flavor chewable tablet and preparing method thereof
A technology for telmisartan and chewable tablets, applied in the field of telmisartan flavored chewable tablets and preparation thereof, can solve the problems of unsolvable and low bioavailability, and achieves improved stability, simple process, isolation and neutralization reaction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0040] Preparation of coating solution
[0041]Prepare 100ml of 20% ethanol-water solution, add 6g of carboxymethyl ethyl cellulose, 2g of lauric acid, 2g of sodium citrate, 1g of ascorbic acid, and 2g of cyclodextrin, and stir evenly to obtain coating solution A.
[0042] Prepare 100ml of 20% ethanol-water solution, add 6g of hydroxypropyl methylcellulose, 2g of lauric acid, 2g of sodium citrate, 1g of ascorbic acid, and 2g of cyclodextrin, and stir evenly to obtain coating solution B.
preparation example 2
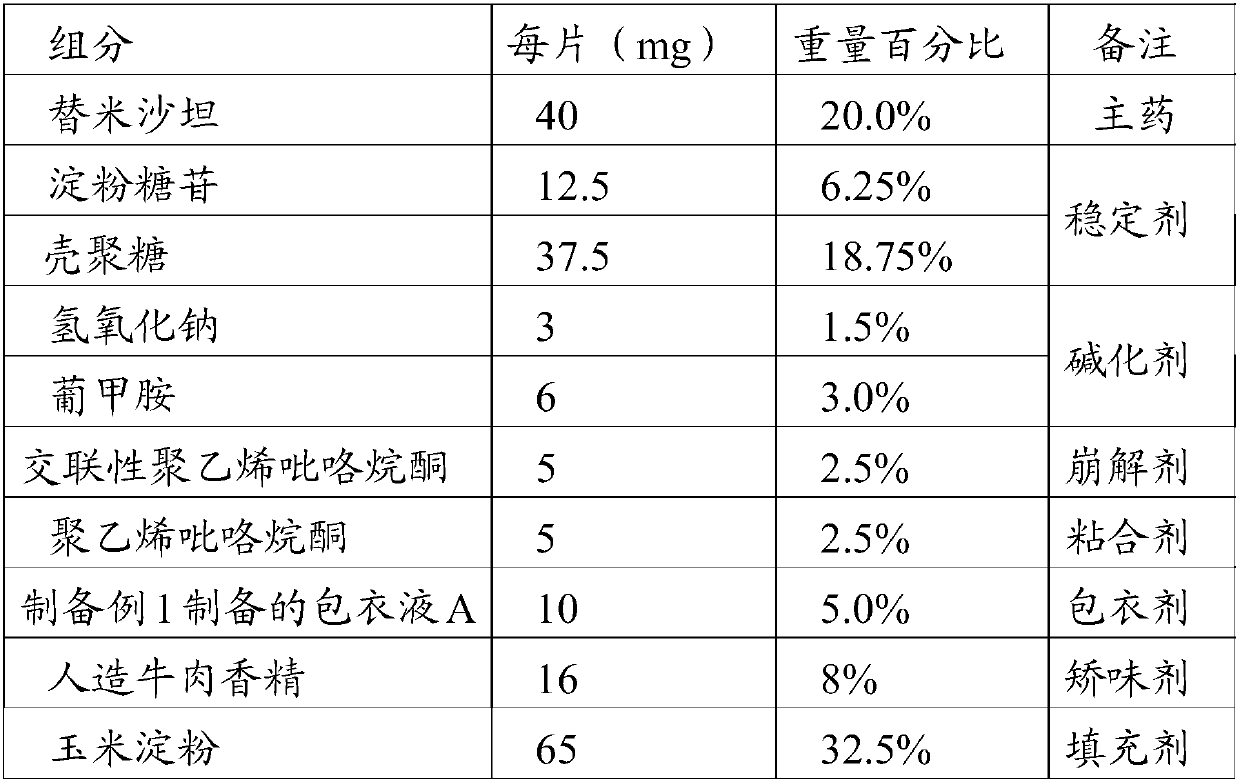
[0044] This embodiment prepares the telmisartan flavor chewable tablet of the present invention, is made by the raw material of percentage by weight as in Table 1:
[0045] Table 1 Components and contents of telmisartan flavor chewable tablets of the present invention
[0046]
[0047] Preparation method: pass telmisartan, sodium hydroxide, meglumine, starch glycoside, chitosan, cornstarch, cross-linked polyvinylpyrrolidone and artificial beef essence through 80-mesh sieve respectively, and take the sieved product in proportion Mix telmisartan, sodium hydroxide, meglumine, starch glucoside, chitosan, and cross-linked polyvinylpyrrolidone evenly, use 5% polyvinylpyrrolidone aqueous solution to make a soft material, and pass through a 40-mesh sieve granules, dried at 40°C, passed through 40 mesh and 65 mesh sieves for granulation, and removed coarse particles and fine powders to obtain granules; the above granules were fluidized bed coated with the coating solution A prepared...
preparation example 3
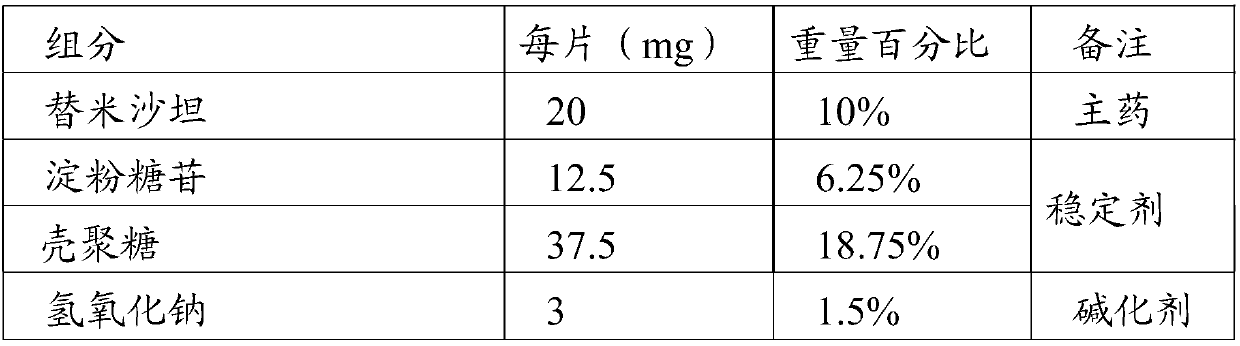
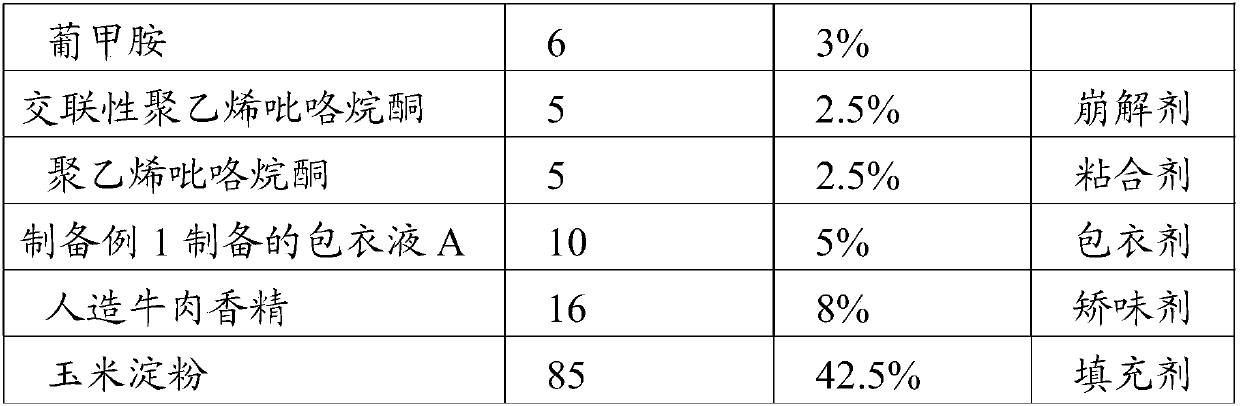
[0049] This embodiment prepares the telmisartan flavor chewable tablet of the present invention, is made of the raw material such as table 2 weight percent:
[0050] Table 2 Components and contents of telmisartan flavor chewable tablets of the present invention
[0051]
[0052]
[0053] Preparation method: pass telmisartan, sodium hydroxide, meglumine, starch glycoside, chitosan, cornstarch, cross-linked polyvinylpyrrolidone and artificial beef essence through 80-mesh sieve respectively, and take the sieved product in proportion Mix telmisartan, sodium hydroxide, meglumine, starch glucoside, chitosan, and cross-linked polyvinylpyrrolidone evenly, use 5% polyvinylpyrrolidone aqueous solution to make a soft material, and pass through a 40-mesh sieve granules, dried at 40°C, passed through 40 mesh and 65 mesh sieves for granulation, and removed coarse particles and fine powders to obtain granules; the above granules were fluidized bed coated with the coating solution A pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com