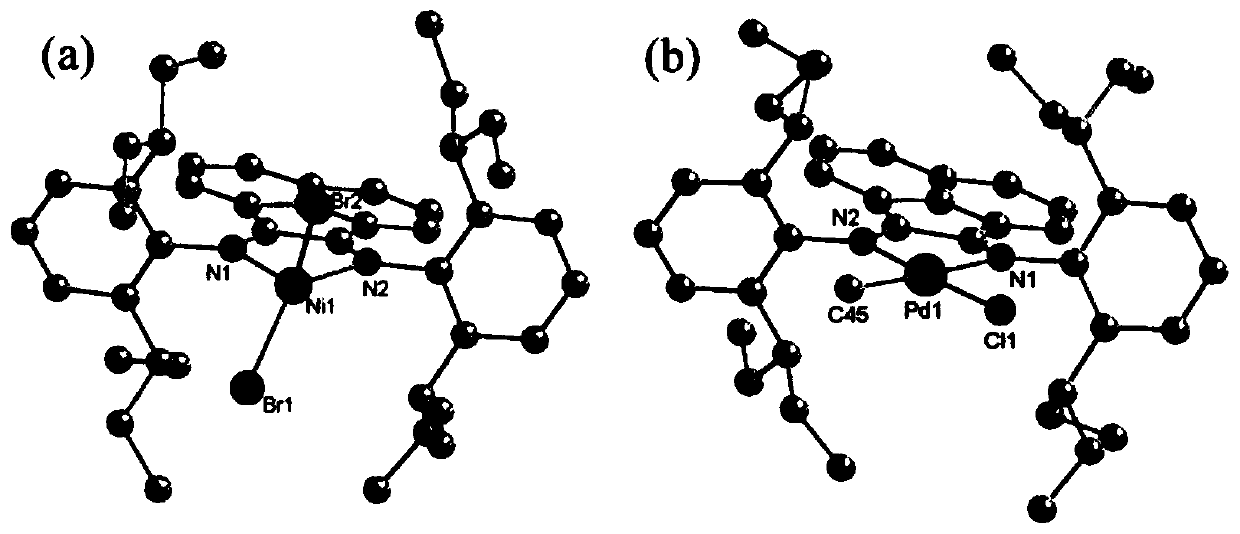
Large-steric-hindrance flexible diimine ligand, diimine nickel and palladium complexes based on large-steric-hindrance flexible diimine ligand and catalytic application of diimine nickel and palladium complexes
A technology of diimine ligand and diimine palladium, which is applied in the preparation of imino compounds, palladium organic compounds, nickel organic compounds, etc., can solve the problems of increasing the activation energy of ethylene monomer, disadvantages, etc., and achieve good catalytic activity, Excellent elastic recovery performance, the effect of good recovery performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] In this example, the large steric hindrance flexible diimine ligand L1 is prepared, and its structure is as follows:
[0042]
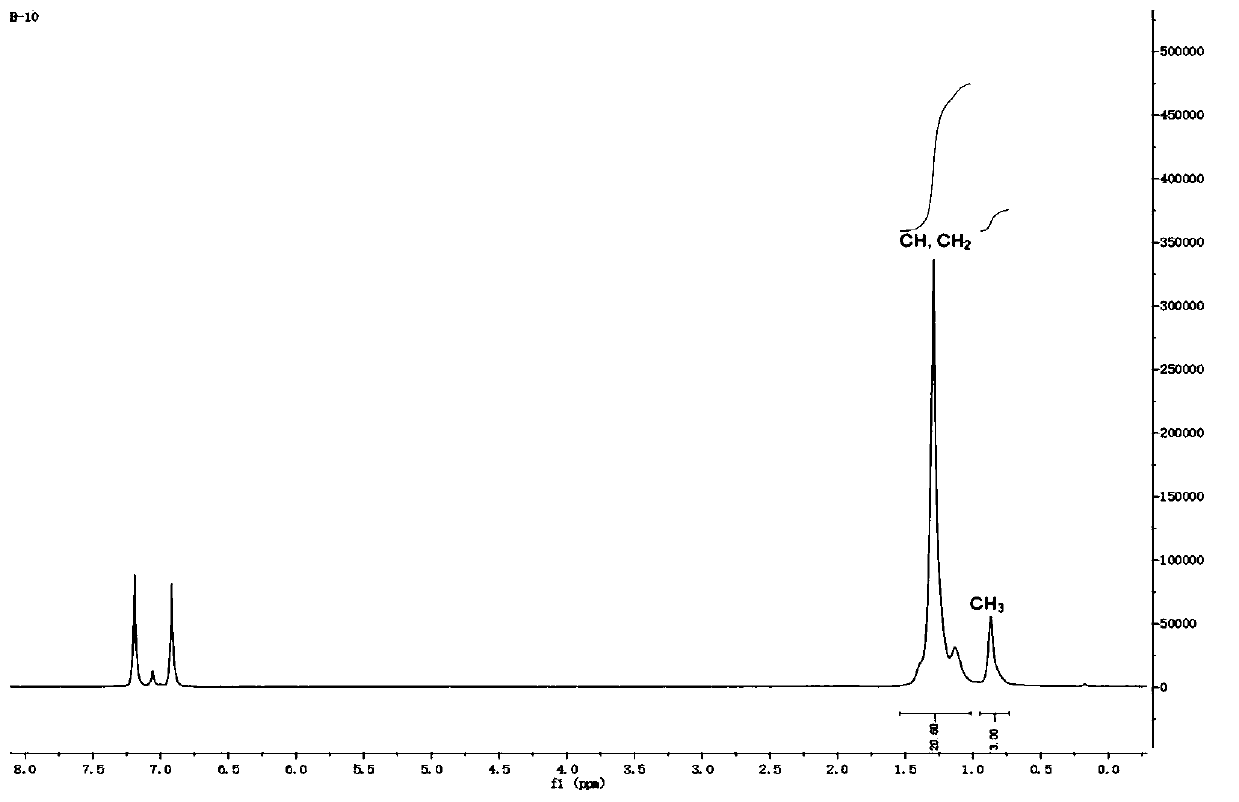
[0043]The synthesis process is as follows: under a nitrogen atmosphere, acenaphthoquinone (0.55g, 3mmol, 1.0eq) and 2,6-di(pent-3-yl)aniline (1.54g, 6.6mmol, 2.2eq) were suspended in acetonitrile (40mL ) and acetic acid (16 mL), then mixed. The mixture was vigorously stirred at 90 °C for 12 hours, turning into a reddish-brown solution. Subsequently, the solution was cooled to room temperature, and a yellow precipitate was collected by filtration. The solid was washed with acetonitrile and dried under vacuum to obtain L1 as a yellow powder with a yield of 74% (1.36 g). 1 H NMR (500MHz, CDCl 3 )δ7.84(d, J=8.2Hz, 2H, Ar-H), 7.32(t, J=7.7Hz, 2H, Ar-H), 7.19(m, 6H, Ar-H), 6.68(d, J=7.1Hz,2H,Ar-H),2.62(m,4H,CH),1.66(m,4H,CH 2 ),1.57(m,4H,CH 2 ),1.48(m,4H,CH 2 ),1.40(m,4H,CH 2 ),0.83(s,12H,CH 3 ),0.52(s,12H,CH 3 ). 13 C NMR (126MHz, CDCl...
Embodiment 2
[0045] This example prepares the large steric hindrance flexible diimine ligand L2, its structure is as follows:
[0046]
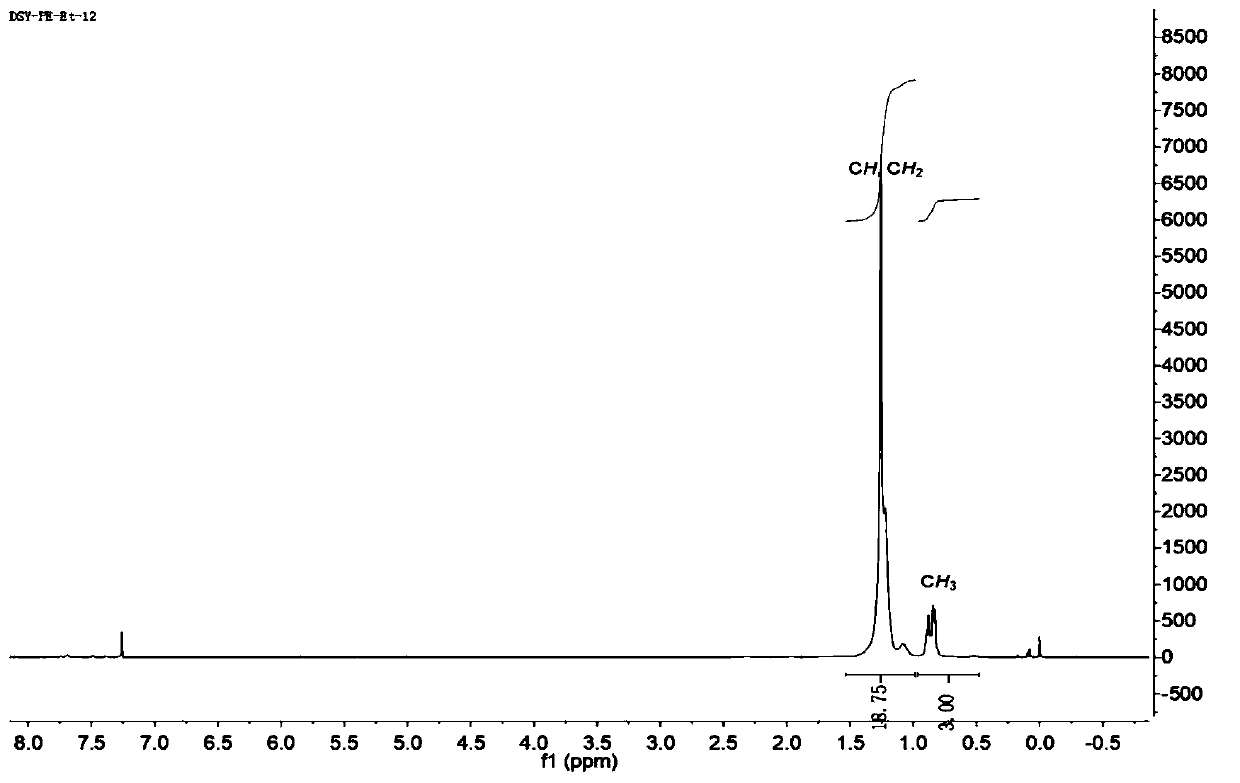
[0047] The same method was used for the synthesis of L1, except that 2,6-bis(hept-4-yl)aniline (1.91 g, 6.6 mmol, 2.2 equiv) was used. L2 was obtained as a yellow powder in a yield of 71% (1.54 g). 1 H NMR (500MHz, CDCl 3 )δ7.82(d,J=8.2Hz,2H,Ar-H),7.33–7.29(m,2H,Ar-H),7.19–7.14(m,6H,Ar-H),6.66(d,J =7.2Hz,2H,Ar-H),2.79–2.73(m,4H,CH),1.54(ddd,J=16.2,9.3,5.6Hz,4H,CH 2 ), 1.49–1.38 (m, 8H, CH 2 ), 1.39–1.30 (m, 4H, CH 2 ),1.30–1.15(m,8H,CH 2 ), 0.91 (tdd, J=13.9, 11.2, 6.6Hz, 8H, CH 2 ),0.80(t,J=7.3Hz,12H,CH 3 ),0.34(t,J=7.3Hz,12H,CH 3 ). 13 C NMR (126MHz, CDCl 3 ( z): Calculate C 52 h 73 N 2 :725.5774,actually measured,725.5754,[M+H] + .
Embodiment 3
[0049] In this example, the large steric hindrance flexible diimine ligand L3 is prepared, and its structure is as follows:
[0050]
[0051] The same method was used for the synthesis of L1, except that 2,6-bis(non-5-yl)aniline (2.28 g, 6.6 mmol, 2.2 eq.) was used. The obtained L3 was a yellow powder with a yield of 96.0% (2.41 g). 1 H NMR (500MHz, CDCl3): δ7.81(d, J=8.2Hz, 2H, Ar-H), 7.30(t, J=7.7Hz, 2H, Ar-H), 7.20–7.14(m, 6H Ar -H),6.67(m,2H,Ar-H),2.76–2.69(m,4H,CH),1.62–1.55(m,4H,CH 2 ),1.44(m,8H,CH 2 ), 1.39–1.32 (m, 4H, CH 2 ),1.27–1.20(m,8H,CH 2 ),1.18–1.12(m,6H,CH 2 ),0.91–0.71(m,30H,CH 2 ,CH 3 ),0.35(t,J=6.6Hz,12H,CH 3 ). 13 C NMR (126MHz, CDCl 3 ): δ160.70 (C=N), 150.25, 140.96, 133.67, 131.20, 130.15, 128.73, 127.64, 124.92, 124.34, 123.78, 39.94, 36.69, 34.92, 30.40, 29.82, 13.44, ES, 343.94 -MS(m / z): Calculate C 60 h 89 N 2 :837.7026,actually measured,837.7012,[M+H] +
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com