Method for synthesizing phenylserine derivative based on fixed bed reactor
A fixed-bed reactor, a technology of phenylserine, which is applied in the field of synthesis of phenylserine derivatives, can solve the problems of high pollution, low economy, non-compliance with environmental protection policies, etc., and achieves reduction of environmental pollution, labor and time, and reduction of The effect of emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
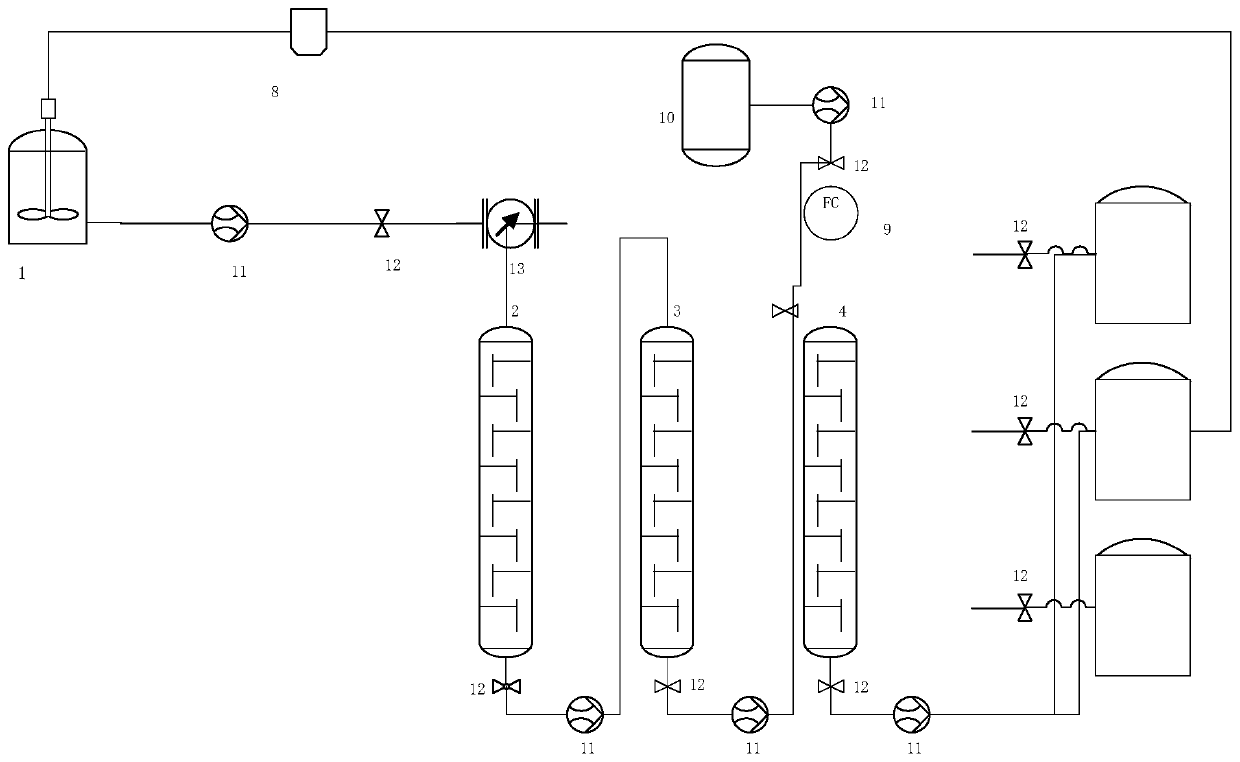
[0035] like figure 1 Shown is a fixed bed reaction device for synthesizing phenylserine derivatives, including a premixer 1, the premixer 1 is connected with the fixed bed 2, and the connected pipeline is equipped with a liquid pump 11 and a control valve 12; the lower outlet of the fixed bed 1 ( or called the outlet) is connected to the inlet of the first separator 3, and the connected pipeline is equipped with a liquid pump 11 and a control valve 12; the outlet of the first separator 3 is connected to the inlet of the second separator 4, and the connected pipeline is equipped with Liquid pump 11 and control valve 12, water storage tank 10 and the inlet of the second separator 4 are connected, and the connected pipeline is equipped with liquid pump 11, control valve 12 and flow meter 13 (or called flow controller); the second separator 4 They are respectively connected to the first storage tank 5, the second storage tank 6, and the third storage tank 7, and each connecting pi...
Embodiment 2
[0037] Adopt the synthetic method of the p-thiamphenicol phenylserine of the fixed-bed reactor of embodiment 1, flow process is as follows:
[0038] Each reactor is filled with catalyst and filler in advance. The fixed bed catalyst is copper ion fixed on the chelating resin through complexation. The resin is LSC-910 chelating resin from Lanxiao, and the copper ion loading is 1.5 %; The metal ion adsorption resin is Samyang CLR-08 chelating resin; the adsorption separation resin is Purite C115E resin, and purified water is used to pass through the equipment, the equipment and fillers are thoroughly cleaned, and the washing water is detected by HPLC (230nm absorption wavelength, isocratic elution) to no obvious impurities (50 times the volume of washing water to dissolve the standard, wherein the maximum impurity introduced in the washing water is <0.1%);
[0039] Start the premixer, add raw materials (ie: 4-thiamphenicol benzaldehyde, glycine, phosphate buffered saline, water),...
Embodiment 3
[0043] Adopt the synthetic method of the p-thiamphenicol phenylserine of the fixed-bed reactor of embodiment 1, flow process is as follows:
[0044] Each reactor is filled with catalyst and filler in advance. The fixed bed catalyst is copper ion fixed on the chelating resin through complexation. The resin is LSC-910 chelating resin from Lanxiao, and the copper ion loading is 1.5 %, the metal ion adsorption resin is LSC-910 chelating resin from Lanxiao, the adsorption separation resin is Purite C115E resin, and purified water is used to pass through the equipment, the equipment and packing are thoroughly cleaned, and the washing water is detected by HPLC (230nm absorption wavelength, isocratic elution) to no obvious impurities (50 times the volume of washing water to dissolve the standard, wherein the maximum impurity introduced in the washing water is <0.1%);
[0045] Start the premixer, add the materials p-thymphenylbenzaldehyde, glycine, phosphate buffer salt, and purified w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com