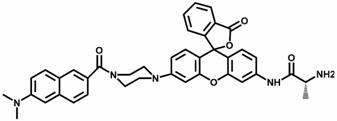
A two-photon ratiometric fluorescent probe compound for aminopeptidase n detection and its preparation method
A technology for fluorescent probes and compounds, applied in the field of fluorescent sensor probe compounds, can solve the problems of lack of specificity and sensitivity, failure to provide real-time information, poor spatial resolution, etc., and achieve a wide range of applications and strong anti-interference ability. , the effect of a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A method for preparing a fluorescent probe compound for aminopeptidase N detection, the steps comprising:
[0040] 1) Synthesis of Compound 1:
[0041] Dissolve 6-amino-2-naphthoic acid (30 mmol, 1 eq) in 15 mL of glacial acetic acid and 100 mL of 37% aqueous formaldehyde, then add cyanide dissolved in methanol solution to the mixture under stirring in an ice-water bath Sodium borohydride (90mmol, 3 eq), stirred at room temperature for 3 h, the methanol solution was evaporated, the remaining mixture was diluted with brine, and the pH was adjusted to 6. It was extracted with ethyl acetate, dried over anhydrous sodium sulfate, evaporated under reduced pressure, and recrystallized to obtain compound 1 as a light brown solid with a yield of 80%.
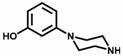
[0042] 2) Synthesis of Compound 2:
[0043] To 25 mL of 3-aminophenol (10 mmol, 1 eq) in ethylene glycol was added bis(2-chloroethyl)amine hydrochloride (12 mmol, 1.2 eq) and the reaction mixture was stirred at 125 °C overnight ...
Embodiment 2
[0055] The I of probe compound FTP-N with different APN concentrations (0-90 ng / mL) 540 nm / I 452 nm Ratiometric Fluorescence Emission Changes
[0056] Take the FTP-N synthesized in Example 1 to prepare a 5 μM probe solution, add different amounts (0-90 ng / mL) of APN standard solution, and measure its fluorescence properties. Using 375 nm as the excitation light, as the amount of APN increases, the fluorescence intensity at 452 nm decreases sharply, and a red-shifted fluorescence peak appears at 540 nm, providing a visualized ratiometric fluorescence signal (I 540 nm / I 452 nm ).
Embodiment 3
[0058] Measurement of Fluorescent Linear Range of Compound FTP-N Fluorescent Probe
[0059] Take the fluorescent probe solution (5 μM) in Example 2, add APN (0-5 ng / mL) respectively, and perform fluorescence detection (λ ex =375 nm), indicating that the probe has a linear relationship in the range of APN concentration 0-5 ng / mL, and the linear correlation coefficient is R 2 =0.99823.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com