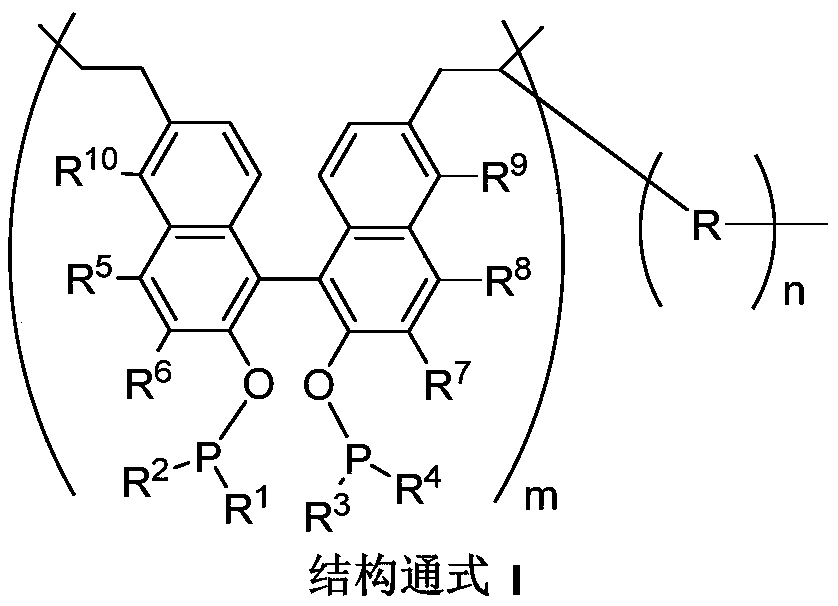
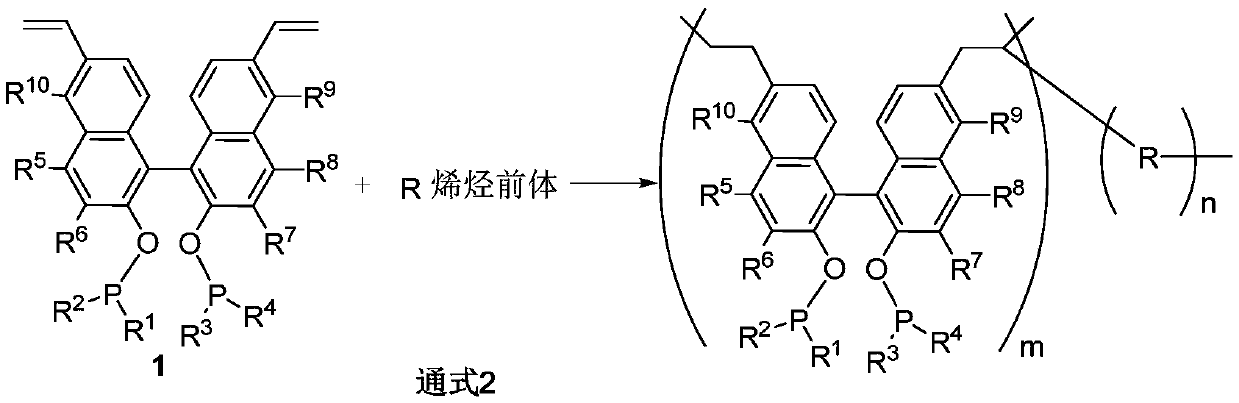
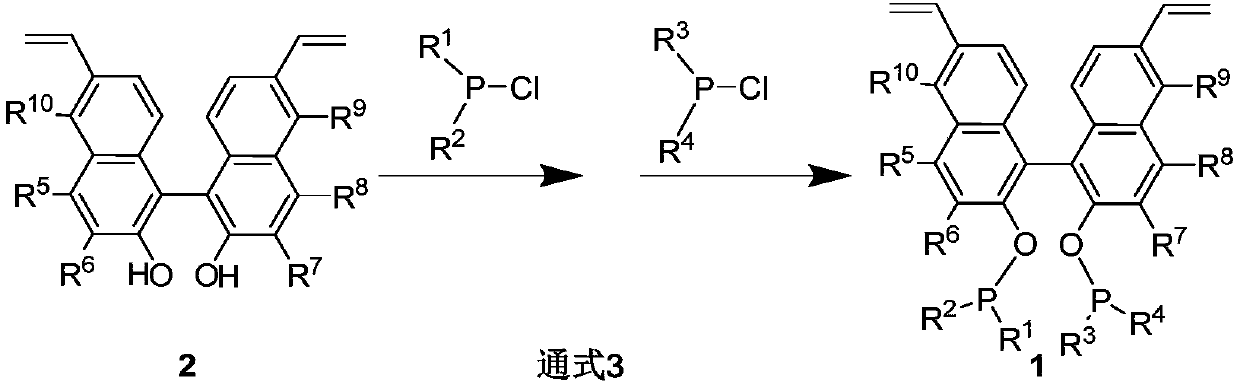
First-class porous organic polymer containing phosphine ligand as well as preparation method and application of first-class porous organic polymer
A technology for phosphine ligands and polymers is applied in the field of porous polymers containing bidentate phosphoramidite phosphine ligands and their preparation, which can solve the problems of easy generation of hydrogenated products and low catalyst activity, and achieve good selectivity and recyclability. , the preparation method is simple, the effect of high catalytic rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The vinyl binaphthol used in the following examples is prepared by the following method, specifically:
[0042] Vinyl binaphthol preparation of
[0043] Under nitrogen protection, add raw materials to the reaction flask 500mg, C 2 h 3 BF3 K (664 mg, 4.9 mmol), THF (6 mL) and water (1 mL), heated to reflux for 24 hours, and the product was separated and purified by column chromatography. The obtained product (200 mg), methanol (6.2 mL), water (3.1 mL) and saturated NaHCO 3 (6.2 mL) was further added into the reaction flask, and the mixture was heated to 50° C. and reacted for 5 hours. The obtained crude product was separated and purified by column chromatography to obtain vinyl biphenol. The product is characterized as follows: 1 H NMR (500MHz, CDCl 3 )δ7.95(d, J=10.0Hz, 2H), 7.83(s, 2H), 7.49(d, J=10.0Hz, 2H), 7.39(d, J=10.0Hz, 2H), 7.13(d, J=10.0Hz, 2H), 6.88(dd, J=20.0, 10.0Hz, 2H), 5.82(d, J=20.0Hz, 2H), 5.32(d, J=10.0Hz, 2H), 5.16(s, 2H)ppm.
Embodiment 2
[0045] synthesis
[0046] Under argon atmosphere, add dipyrrole phosphorus chloride (131.0mg, 1.9mmol), anhydrous triethylamine (0.09mL, 0.69mmol) and anhydrous tetrahydrofuran (6mL) respectively into a 100mL Schlenk tube, cool to 0°C , add dropwise 6,6'-divinyl-2,2'-binaphthyldiol (112mg, 0.35mmol) anhydrous tetrahydrofuran (6mL) solution, slowly warming up to room temperature and stirring overnight, stop the reaction, spin off the solvent under reduced pressure, column chromatography (eluent: ethyl acetate: petroleum ether = 1 / 10) 203.3 mg of a colorless oil was isolated with a yield of 88%. 1 H NMR (CDCl 3 ,500MHz): δ7.92(d,J=7.0Hz,2H),7.87(s,2H),7.33(d,J=8.0Hz,2H),7.07(s,2H),7.24-7.20(m, 2H), 6.96-6.90(m, 2H), 6.58(d, J=25.0Hz, 8H), 6.20(d, J=25.0Hz, 8H), 5.88(d, J=17.0Hz, 2H), 5.39( d,J=11.5Hz,2H)ppm. 13 C NMR (125MHz, CDCl 3 ): δ149.2(d, J=12.0Hz), 136.4, 134.5, 133.2, 130.9, 130.5, 126.3, 126.0, 124.5, 122.5, 121.0(d, J=14.4Hz), 120.9(d, J=13.9Hz ), 119.5(d, J...
Embodiment 3
[0048] Polymer I 1 synthesis
[0049] Under a nitrogen atmosphere, anhydrous tetrahydrofuran (11.0 mL) was added to a 50 mL stopcock bottle, and the copolymerization unit (225.0mg, 0.34mol) and (460.0 mg, 1.36 mmol). Finally 25.0 mg of initiator AIBN was added. After stirring at room temperature for 10 min, react at 100°C for 24 h, the product was separated by centrifugation, washed with tetrahydrofuran (3×10 mL) and rotary evaporated to obtain a white solid (640.0 mg).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com