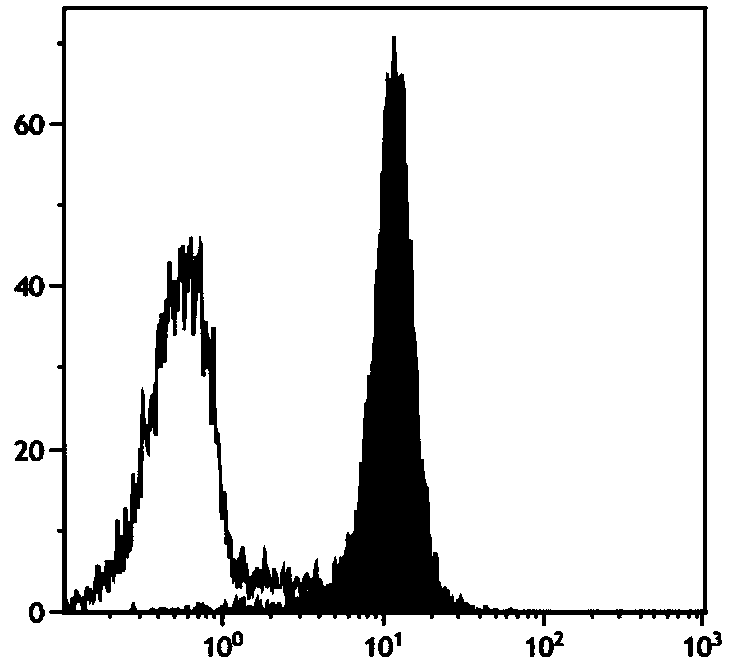
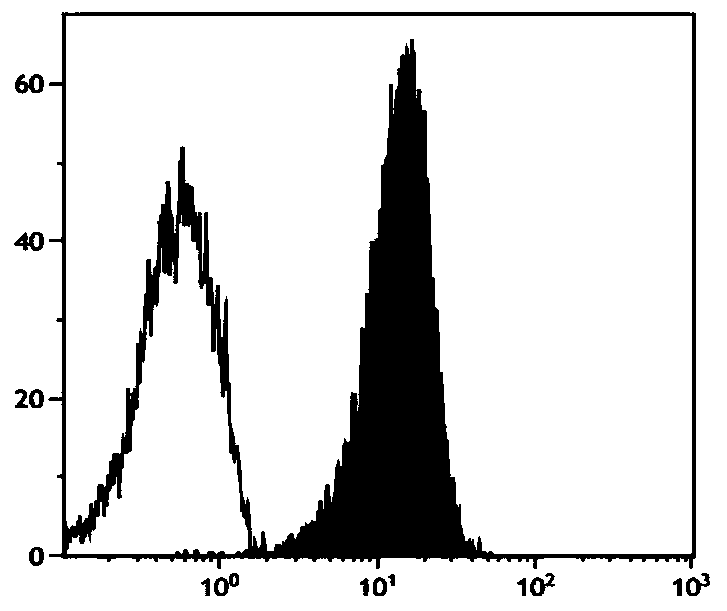
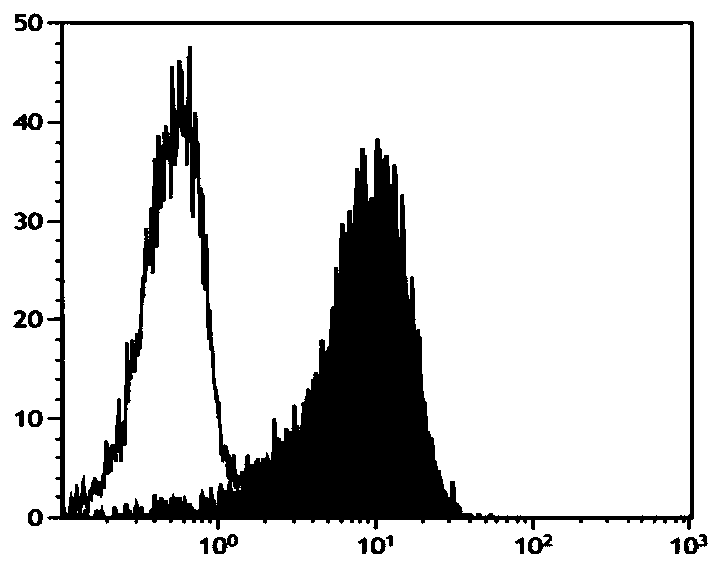
Method for in-vitro large-scale induction of NK cell expansion
A large-scale technology of NK cells, applied in the biological field, can solve the problems of NK cell expansion, artificial antigen-presenting cells complex and cumbersome, etc., and achieve obvious tumor cell killing effect, stable and efficient cytotoxicity, and good cell proliferation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Construction method of artificial antigen-presenting cells CD86CD64mIL-15CD19-K562.
[0055] In the present invention, unless otherwise stated, the percentage symbols represent volume percentages.
[0056] 1. Obtain the target sequence fragment by PCR method
[0057] 1) Obtain CD86, CD64 and mIL-15 genes by using the method of total gene synthesis.
[0058] 2) Primer design, the upstream and downstream primers of the target gene are respectively added with the homologous sequences on both sides of NotI and BamHI on the LV6 vector, which are used for subcloning of the vector. The primer sequences of CD86, CD64 and mIL-15 genes are as follows:
[0059] Table 1 CD64 gene primer sequence
[0060]
[0061]
[0062] Table 2 CD86 gene primer sequence
[0063]
[0064]
[0065]
[0066] Table 3 m-IL15 gene primer sequence
[0067]
[0068]
[0069] 3) The above oligos were synthesized by Shanghai Jima Pharmaceutical Technology Co., Ltd.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com