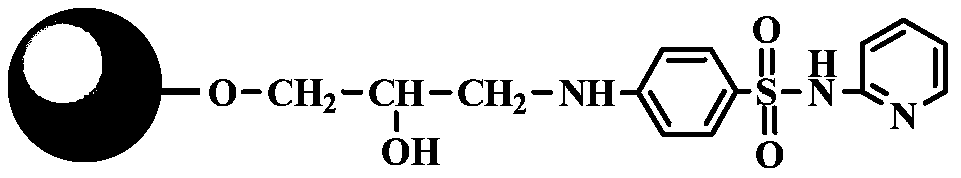Chromatographic medium using aminobenzamide (aminobenzenesulfonamide) pyridine as functional ligand
An amide pyridine and chromatographic medium technology, applied in the field of protein chromatographic separation technology, can solve the problems of affecting the purity of the separated antibody, poor adsorption selectivity, unfavorable separation application, etc., and achieves strong charge induction effect, high ligand density, and adsorption selection. strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Take 10g of dry agarose gel, add 2g 20% (v / v) dimethyl sulfoxide, 1g allyl bromide and 1g sodium hydroxide, activate it in a shaker at 150rpm at 25℃ for 48 hours, filter with suction, The activated matrix was washed with deionized water; then the activated matrix and 1g of N-bromosuccinimide were mixed for bromo-alcoholization, reacted in a shaker at 150 rpm at 25°C for 1 hour, filtered with suction, and washed with deionized water; Mix the brominated substrate with 2g 4-amino-N-(2-pyridyl)benzenesulfonamide and 1M sodium carbonate buffer (pH12), and react in a shaker at 150rpm at 25°C for 48 hours; finally, filter the medium with suction. Wash with ionized water, add to 10g ethanolamine, react in a shaker at 150rpm at 25°C for 12 hours, wash with deionized water to obtain a chromatography medium with 4-amino-N-(2-pyridyl)benzenesulfonamide as ligand , The ligand density is 40μmol / ml.
Embodiment 2
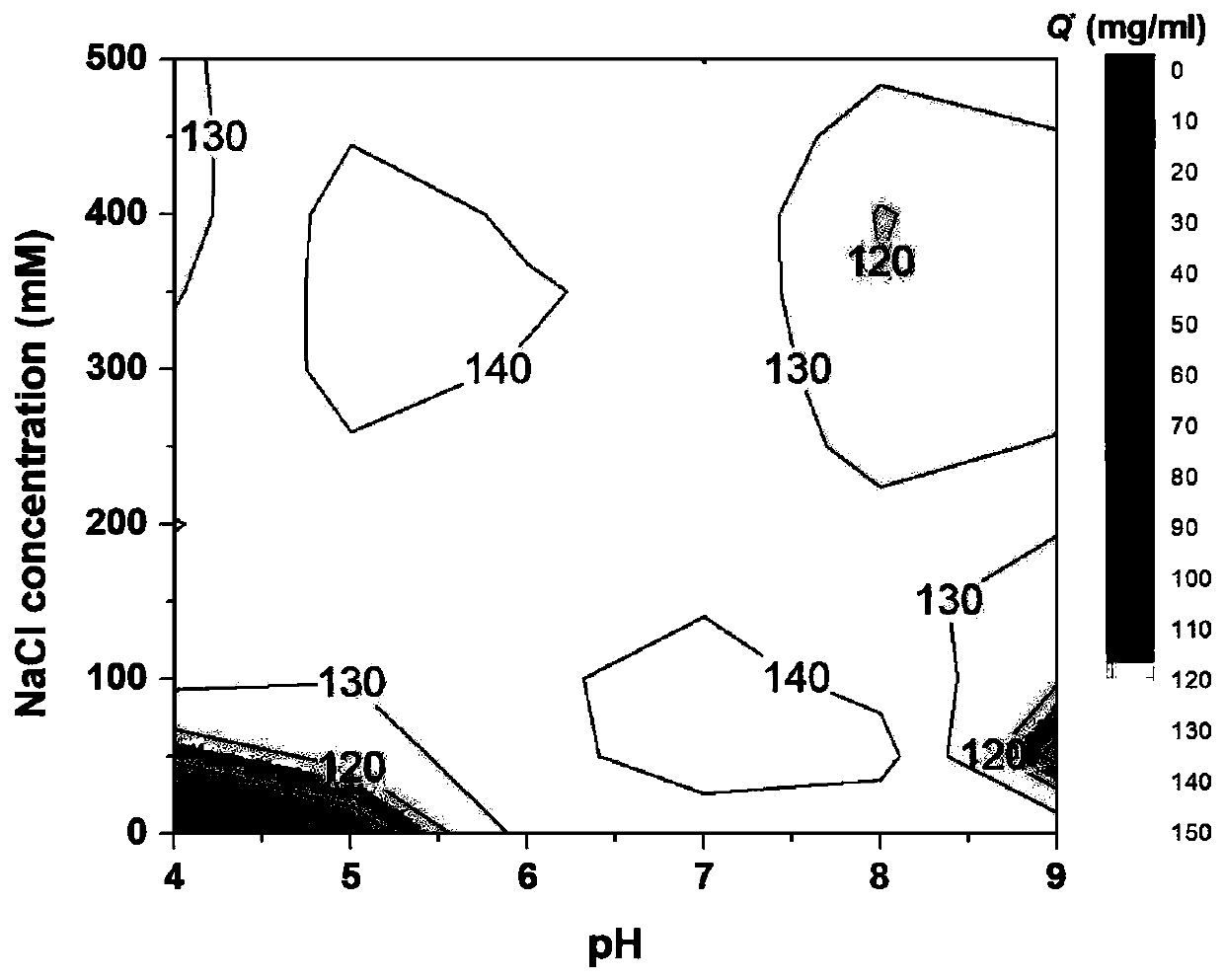
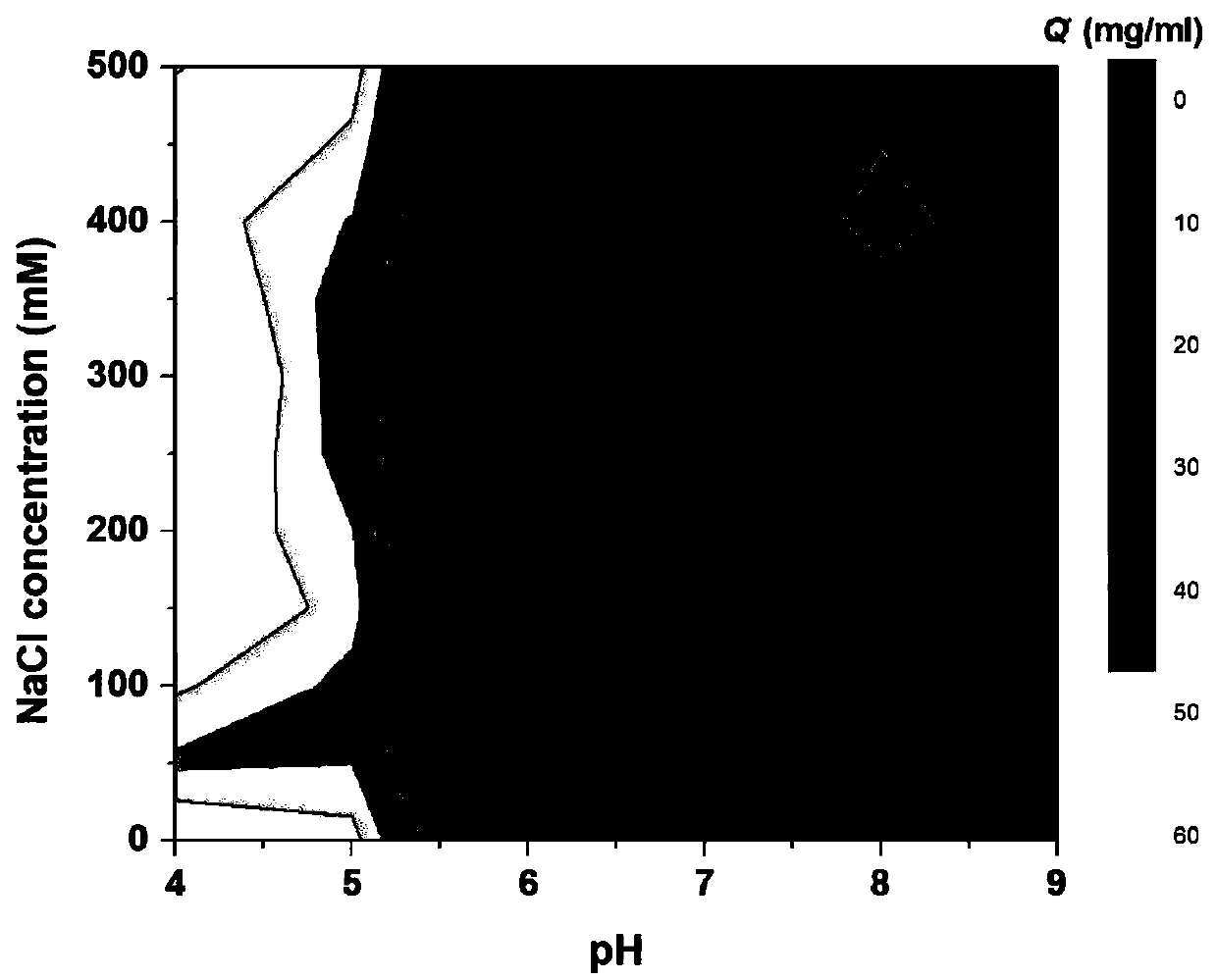
[0031] Take 10g of dry agarose gel, add 10g 20%(v / v) dimethyl sulfoxide, 10g allyl bromide and 5g sodium hydroxide, activate it in a shaker at 150rpm at 25℃ for 8 hours, filter with suction, The activated matrix was washed with deionized water; then the activated matrix and 5g of N-bromosuccinimide were mixed for bromo-alcoholization, reacted in a shaker at 150 rpm at 25°C for 5 hours, filtered with suction, and washed with deionized water; Mix the brominated substrate with 6g 4-amino-N-(2-pyridyl)benzenesulfonamide and 0.5M sodium carbonate buffer (pH 10), and react in a shaker at 150rpm at 25°C for 8 hours; finally, filter the medium with suction. Wash with deionized water, add to 50g ethanolamine, react in a shaker at 150rpm at 25°C for 48 hours, wash with deionized water to obtain a layer with 4-amino-N-(2-pyridyl)benzenesulfonamide as ligand Analysis medium, the ligand density is 150μmol / ml. Under different pH and NaCl concentration, the binding capacity of the medium to ...
Embodiment 3
[0033] Take 10g of dry agarose gel, add 10g 20%(v / v) dimethyl sulfoxide, 10g allyl bromide and 4g sodium hydroxide, activate it in a shaker at 150rpm at 25℃ for 10 hours, filter with suction, The activated matrix was washed with deionized water; then the activated matrix and 5g of N-bromosuccinimide were mixed for bromo-alcoholization, reacted in a shaker at 150 rpm at 25°C for 1 hour, filtered with suction, and washed with deionized water; Mix the brominated substrate with 5g 4-amino-N-(2-pyridyl)benzenesulfonamide and 0.5M sodium carbonate buffer (pH 12), and react in a shaker at 150rpm at 25°C for 16 hours; finally, filter the medium with suction. Wash with deionized water, add to 5g ethanolamine, react in a shaker at 150rpm at 25°C for 4 hours, wash with deionized water to obtain a layer with 4-amino-N-(2-pyridyl)benzenesulfonamide as ligand Analyze the medium and the ligand density is 95μmol / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com