Double-chamber osmotic pump type glipizide controlled release tablet and industrial production method
A technique for glipizide and a production method, which is applied in the field of dual-chamber osmotic pump type glipizide controlled-release tablets and industrialized production, can solve problems such as affecting product quality and yield, affecting product quality, and being difficult to clean, and achieving The effect of reducing equipment investment, eliminating organic residues and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
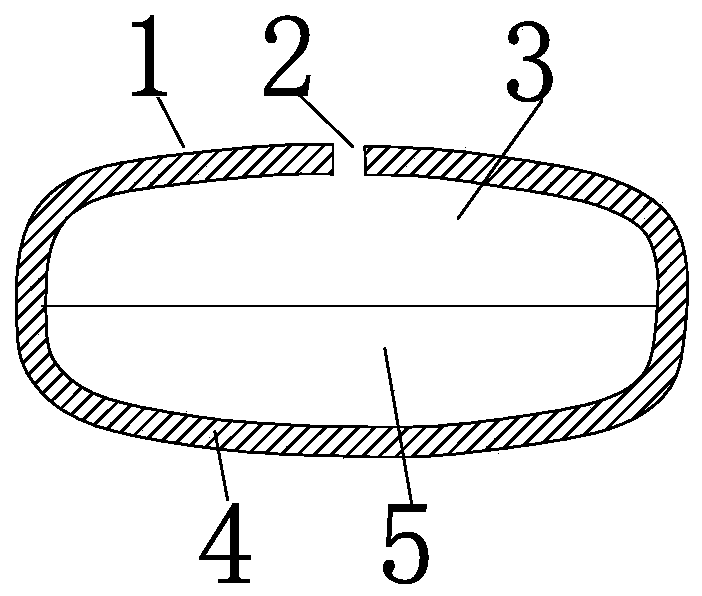
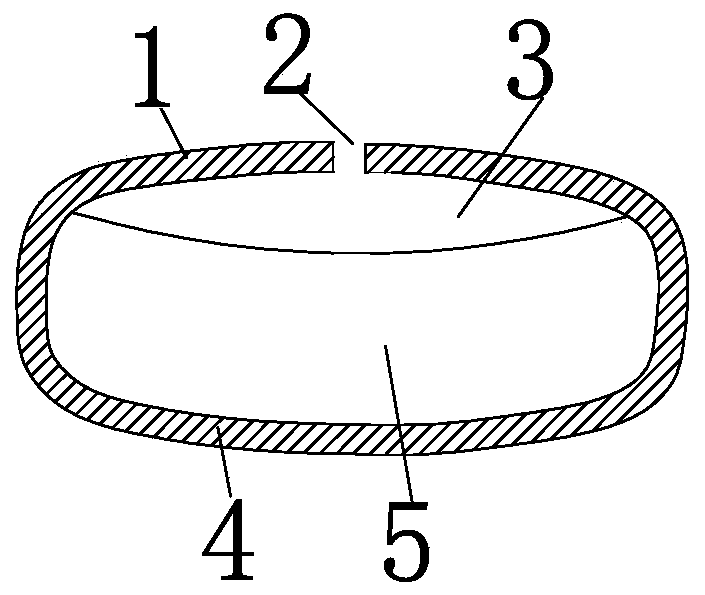
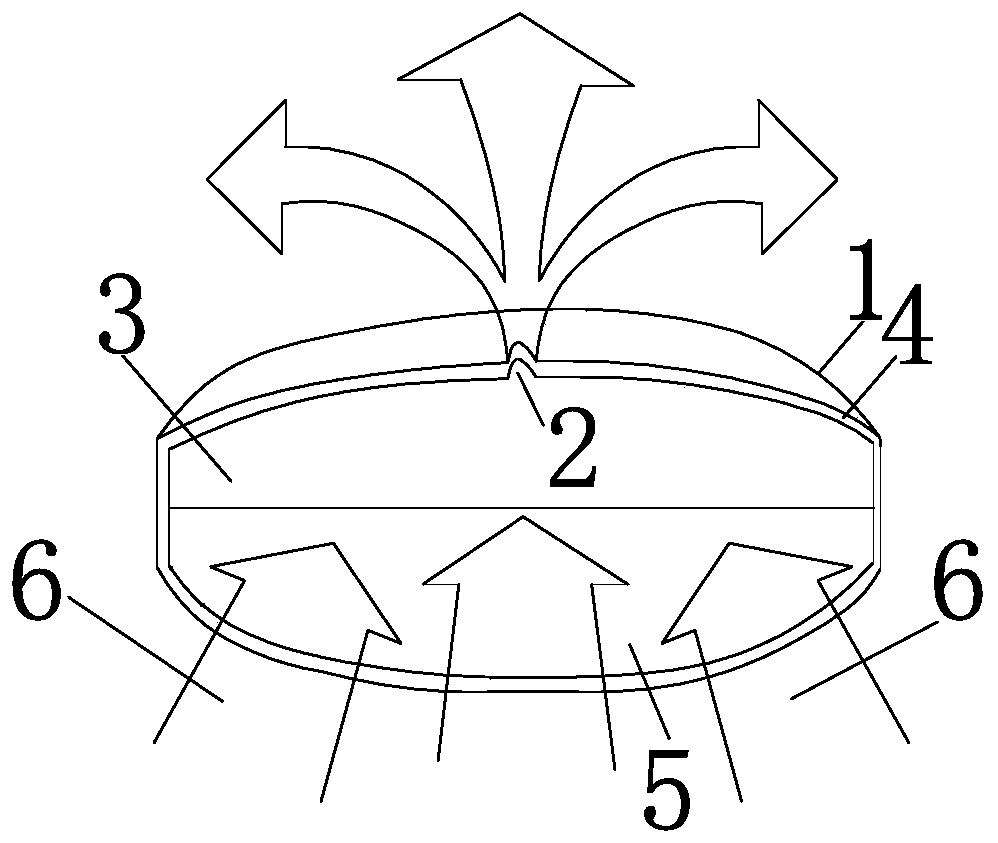
[0109] The double-chamber osmotic pump type glipizide controlled-release tablet of the present invention is composed of a drug layer, a push layer, a rigid semipermeable membrane, drug release holes and a moisture-proof layer; the drug layer uses polyethylene glycol 6000 as a carrier, The hot-melt method was used for granulation without using organic solvents, and the double-chamber osmotic pump glipizide controlled-release tablets were prepared by drawing circular holes with a low-power laser.
[0110] The drug layer is prepared from glipizide, lactose, polyvinylpyrrolidone K90, polyethylene glycol 6000 and magnesium stearate.
[0111] The push layer is prepared from sodium alginate, sodium chloride, polyethylene glycol 6000, iron oxide red, polyvinylpyrrolidone K90 and magnesium stearate.
[0112] Rigid semipermeable membranes are prepared from cellulose diacetate, polyethylene glycol 1500, and acetone.
[0113] The moisture-proof layer is prepared from hydroxypropyl methyl...
Embodiment 2
[0137] The double-chamber osmotic pump type glipizide controlled-release tablet of the present invention is composed of a drug layer, a push layer, a rigid semipermeable membrane, drug release holes and a moisture-proof layer; the drug layer uses polyethylene glycol 6000 as a carrier, The hot-melt method was used for granulation without using organic solvents, and the double-chamber osmotic pump glipizide controlled-release tablets were prepared by drawing circular holes with a low-power laser.
[0138] The drug layer is prepared from glipizide, lactose, polyvinylpyrrolidone K90, polyethylene glycol 6000 and magnesium stearate.
[0139] The push layer is prepared from sodium alginate, sodium chloride, polyethylene glycol 6000, iron oxide red, polyvinylpyrrolidone K90 and magnesium stearate.
[0140] Rigid semipermeable membranes are prepared from cellulose diacetate, polyethylene glycol 1500, and acetone.
[0141] The moisture-proof layer is prepared from hydroxypropyl methyl...
Embodiment 3
[0166] The double-chamber osmotic pump type glipizide controlled-release tablet of the present invention is composed of a drug layer, a push layer, a rigid semipermeable membrane, drug release holes and a moisture-proof layer; the drug layer uses polyethylene glycol 6000 as a carrier, The hot-melt method was used for granulation without using organic solvents, and the double-chamber osmotic pump glipizide controlled-release tablets were prepared by drawing circular holes with a low-power laser.
[0167] The drug layer is prepared from glipizide, lactose, polyvinylpyrrolidone K90, polyethylene glycol 6000 and magnesium stearate.
[0168] The push layer is prepared from sodium alginate, sodium chloride, polyethylene glycol 6000, iron oxide red, polyvinylpyrrolidone K90 and magnesium stearate.
[0169] Rigid semipermeable membranes are prepared from cellulose diacetate, polyethylene glycol 1500, and acetone.
[0170] The moisture-proof layer is prepared from hydroxypropyl methyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com