Cimetidine synthesis process
A synthesis process and cimetidine technology, which is applied in the field of cimetidine synthesis technology, can solve problems such as adverse environmental impact, high synthesis cost, human body harm, etc., achieve less waste, strong reactivity and selectivity, reduce Effects of damage to the human body and the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0039] Below in conjunction with the examples, the specific implementation of the present invention will be further described in detail. The following examples are used to illustrate the present invention, but are not intended to limit the scope of the present invention.
[0040] Cimetidine synthesis technique of the present invention, its operational route is as follows
[0041]
[0042] And, its synthesis process comprises the following steps:
[0043] (1) The first intermediate and the photocatalyst are mixed and dissolved in the reaction solvent;
[0044] (2) and adding the second intermediate to the reaction solution in step (1), after mixing uniformly, a clear solution is obtained;
[0045] (3) Use a light source to irradiate the clear solution obtained in step (2), and cooperate with stirring until the HPLC shows that the first intermediate reacts completely;
[0046] (4) After the reaction finishes, carry out concentration treatment to remove solvent, and use wat...
Embodiment 1
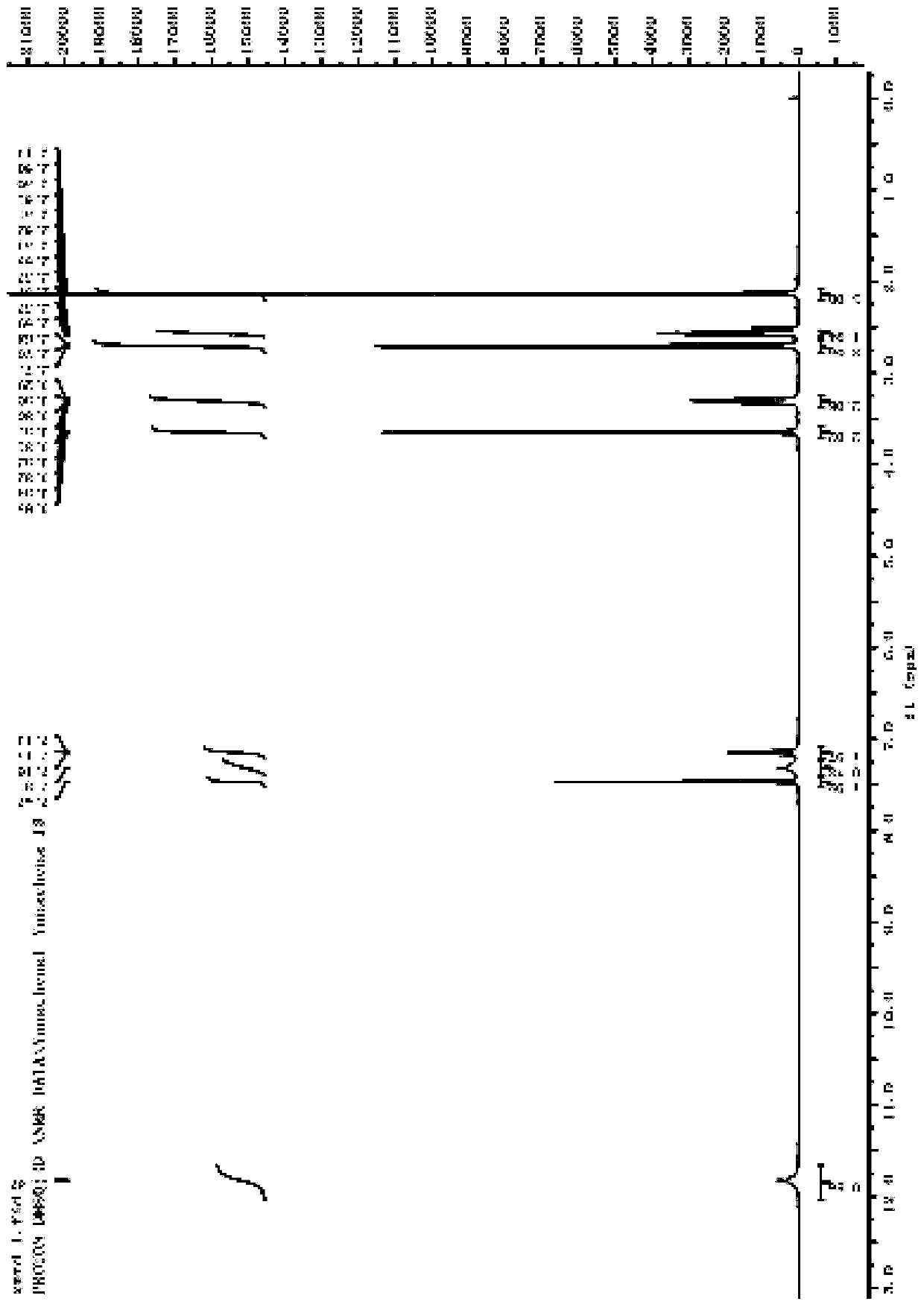
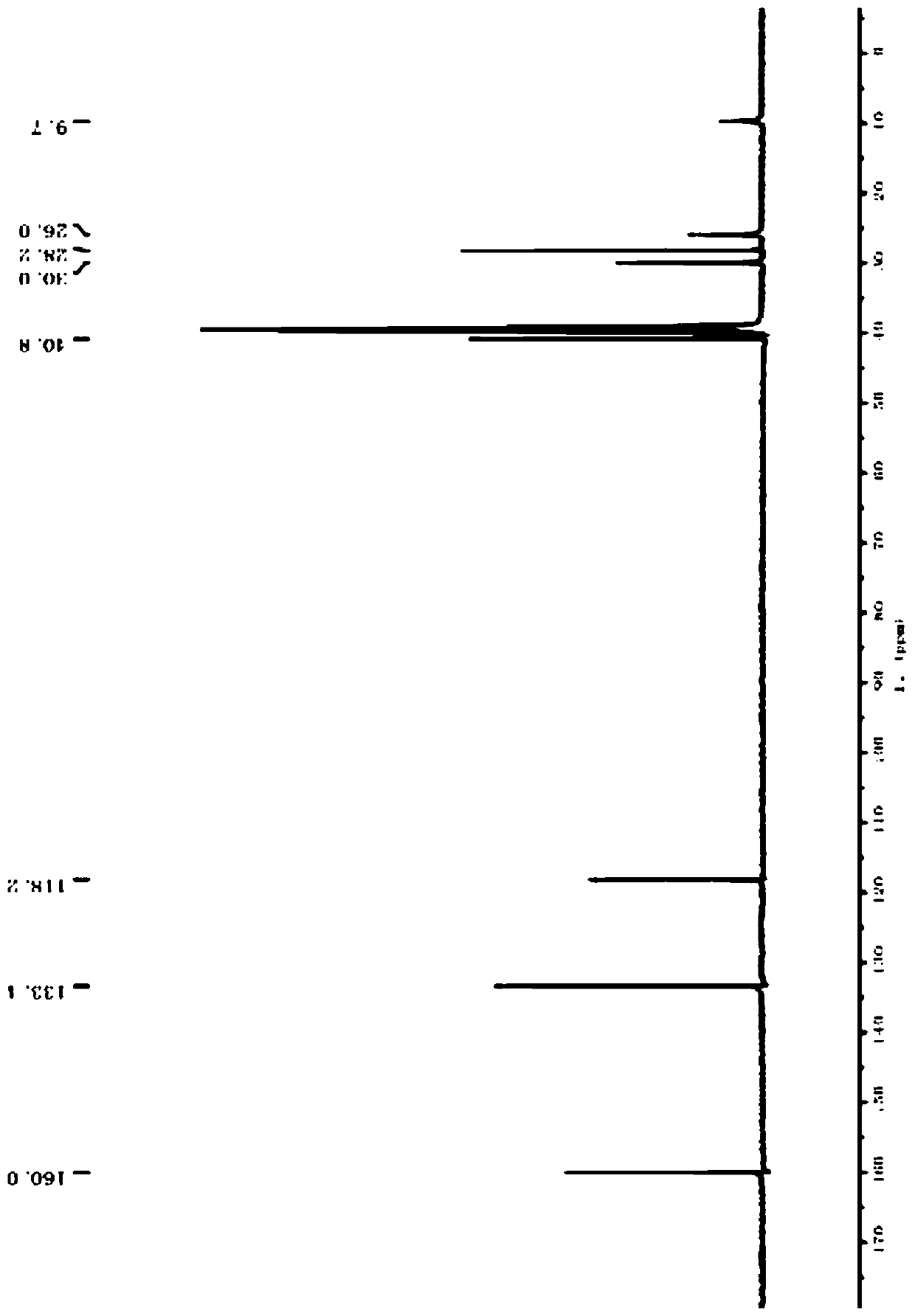
[0048] The first intermediate (0.54g, 2.0mmol) and EosinY (65mg, 0.1mmol) were dissolved in tert-butanol (20mL) and added to thiol (0.47g, 3.0mmol) to obtain a clear solution, which was then illuminated with visible light. Under irradiation, heat to 70°C and stir for 24h. HPLC shows that the first intermediate is completely reacted. After the reaction, wash with saturated sodium bicarbonate (50ml), extract with ethyl acetate (3╳50mL), and combine the organic phases. Dry over anhydrous sodium sulfate, concentrate, and use silica gel column chromatography (dichloromethane / methanol) to obtain cimetidine as a white powder (0.43g, 85%) with a melting point of 139°C to 141°C.
Embodiment 2
[0050] The first intermediate (2.7g, 20mmol) and Ir(ppy) 3 (150mg) was dissolved in ethyl acetate (150mL) and added to disulfide (3.3g, 21mmol) to obtain a clear solution, then, under the irradiation of blue LED light (~390nm), stirring at room temperature for 15h, HPLC It shows that the reaction of the first intermediate is complete. After the reaction, the solvent is concentrated and removed, and cimetidine is recrystallized from isopropanol and water to obtain cimetidine as a white powder (4.9 g, 91%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com