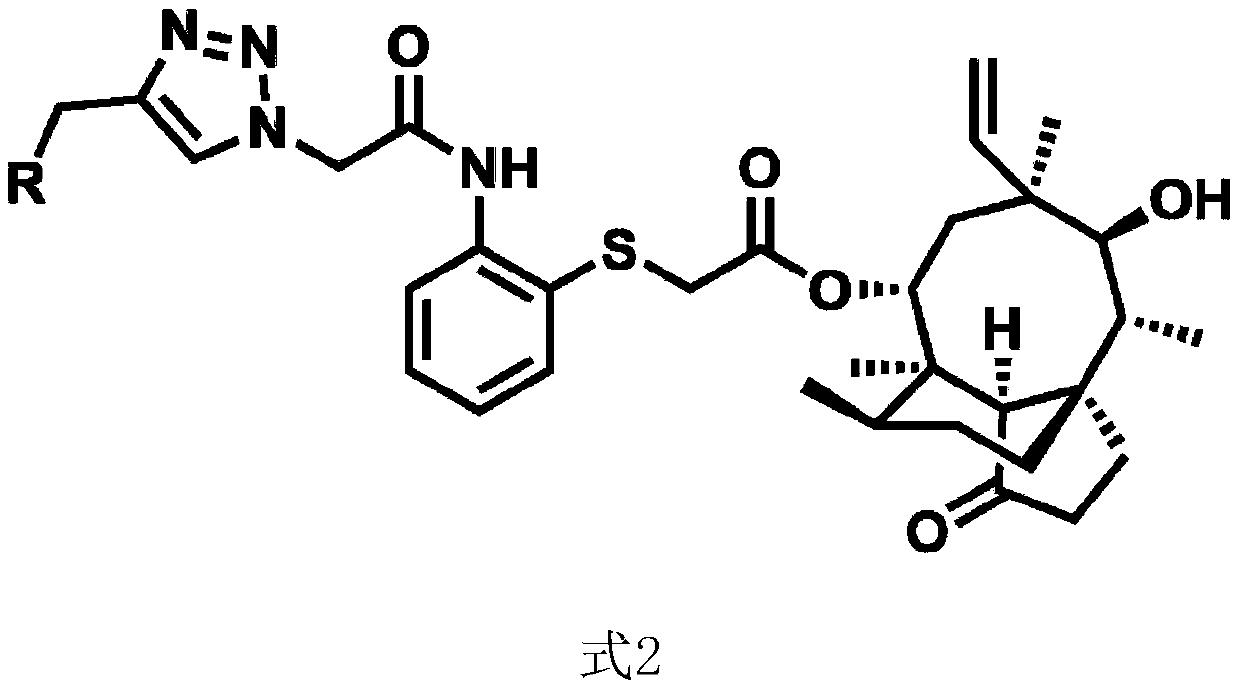
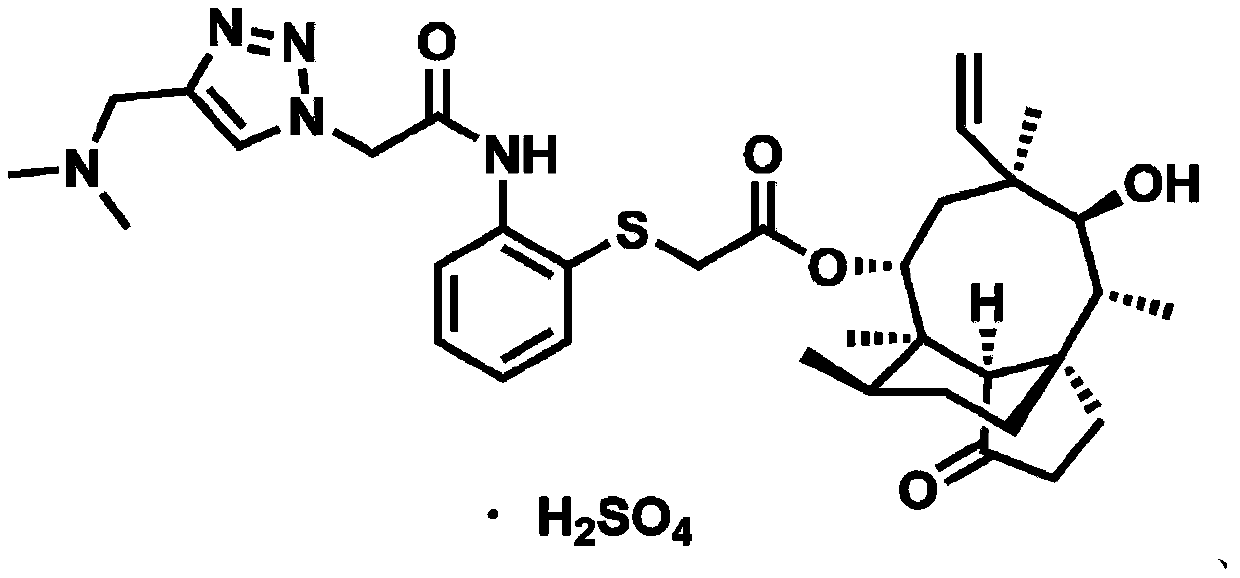
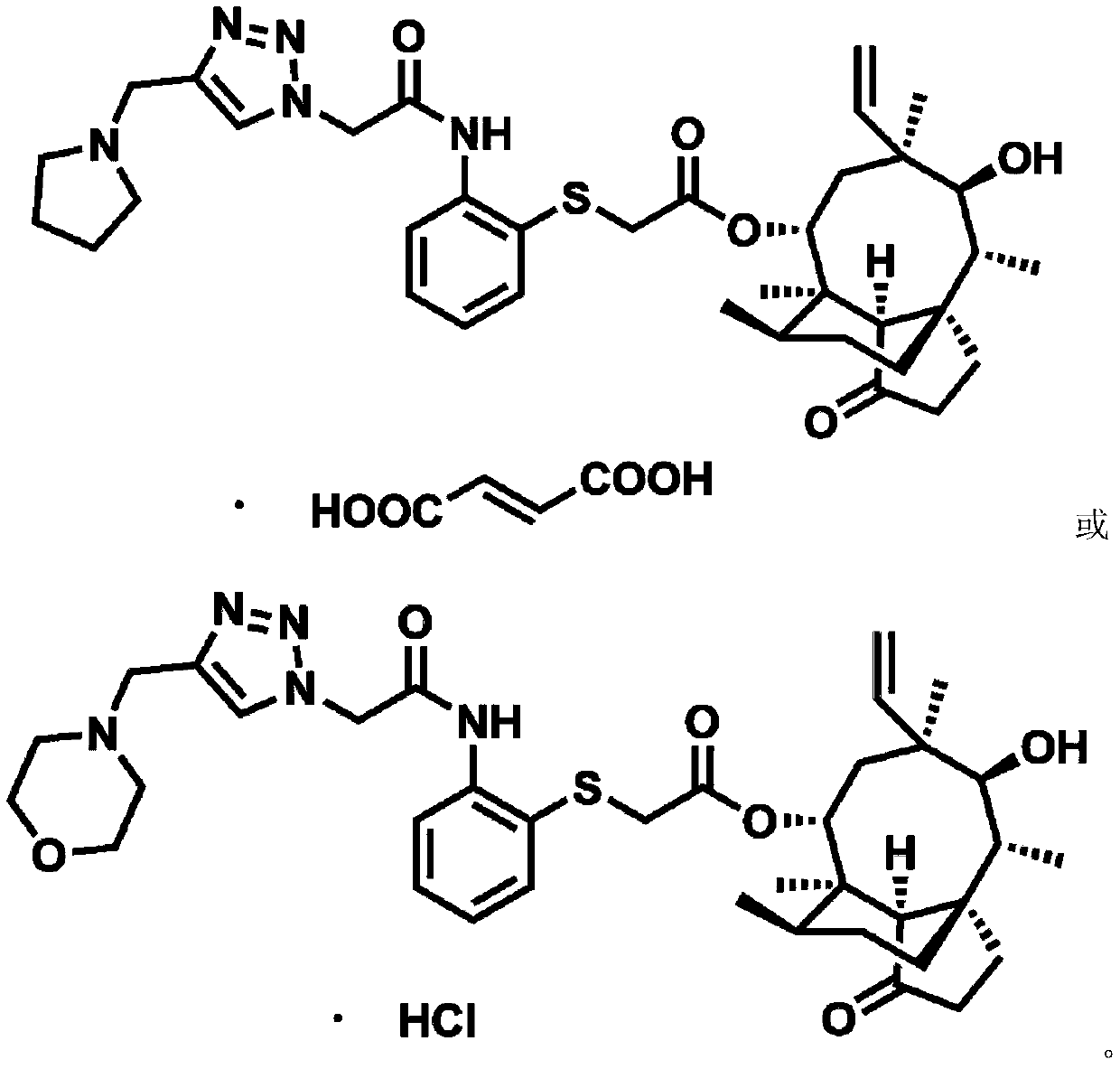
Pleuromutilin derivative with 2-aminothiophenol and 1,2,3-triazole side chain, preparation and application
A technology of aminobenzenethiol and pleuromutilin, applied in medical preparations containing active ingredients, organic chemistry, organic active ingredients, etc., can solve the problem of rare drug-resistant bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Preparation of intermediate Ⅰ: Dissolve 5.4g (14.27mmol) of pleuromutilin in 30ml of pyridine, cool it in ice to about 0°C, add 2.99g (15.70mmol) of p-toluenesulfonyl chloride, keep it warm at 30°C for 2 hours, then add 40ml of ice Quench the reaction with water; pour the reaction solution into a separatory funnel, first add 40ml of chloroform to separate layers, remove the water phase, then wash the organic phase twice with 80ml of sulfuric acid solution with a concentration of 2mol / L, and then wash the organic phase with 30ml of saturated sodium bicarbonate solution Wash the organic phase twice, and finally wash the organic phase twice with 80 ml of deionized water and dry with anhydrous sodium sulfate; dry the organic phase by rotary evaporation, add 15 ml of isopropanol to the residual solid and heat to dissolve, and a large amount of white powder is precipitated after cooling. Suction filtration under reduced pressure, the filtrate was washed with isopropanol, the p...
Embodiment 2
[0096] Example 2 22-O-[2-((4-((diethylamino)methyl)-1H-1,2,3-triazole)acetamido)phenyl]thioacetylmuulin ( Compound 1) Synthesis
[0097]Take 1g (13.67mmol) of diethylamine and dissolve it in 30ml of ethyl acetate, add 3.78g (27.34mmol) of potassium carbonate, then slowly add 1.63g (13.67mmol) of 3-bromopropyne into the reaction system, and keep warm at 20°C Stir the reaction for 3 hours, pour the reaction solution into a separatory funnel, add 40ml of chloroform for extraction, wash twice with aqueous sodium chloride (15% w / v) and dry with anhydrous sodium sulfate, take the organic phase; The intermediate V-1 with the following structure was obtained by rotary evaporation;
[0098]
[0099]
[0100] Take 1g (1.70mmol) of intermediate IV and 0.19g (1.70mmol) of intermediate V-1 and dissolve them in a mixture of 10ml tert-butanol and 10ml water, add 0.0033g (0.068mmol) of copper sulfate pentahydrate and sodium ascorbate 0.0013g (0.068mmol), keep warm at 20°C and stir for...
Embodiment 3
[0101] Example 3 22-O-[2-((4-((morpholinyl)methyl)-1H-1,2,3-triazole)acetamido)phenyl]thioacetylmuulin (compound 2) synthesis
[0102] Take 1.19g (13.67mmol) of morpholine and dissolve it in 30ml of dichloromethane, add 3.78g (27.34mmol) of potassium carbonate, then slowly add 1.63g (13.67mmol) of 3-bromopropyne into the reaction system dropwise, and keep warm at 25°C Stir the reaction for 3 hours, pour the reaction solution into a separatory funnel, add 40ml of chloroform for extraction, wash twice with aqueous sodium chloride (15% w / v) and dry with anhydrous sodium sulfate, take the organic phase; The intermediate V-2 with the following structure was obtained by rotary evaporation;
[0103]
[0104] Take 1g (1.70mmol) of intermediate IV and 0.21g (1.70mmol) of intermediate V-2 and dissolve them in a mixture of 10ml tert-butanol and 10ml water, add 0.0033g (0.068mmol) of copper sulfate pentahydrate and sodium ascorbate 0.0013g (0.068mmol), keep warm at 25°C and stir for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com