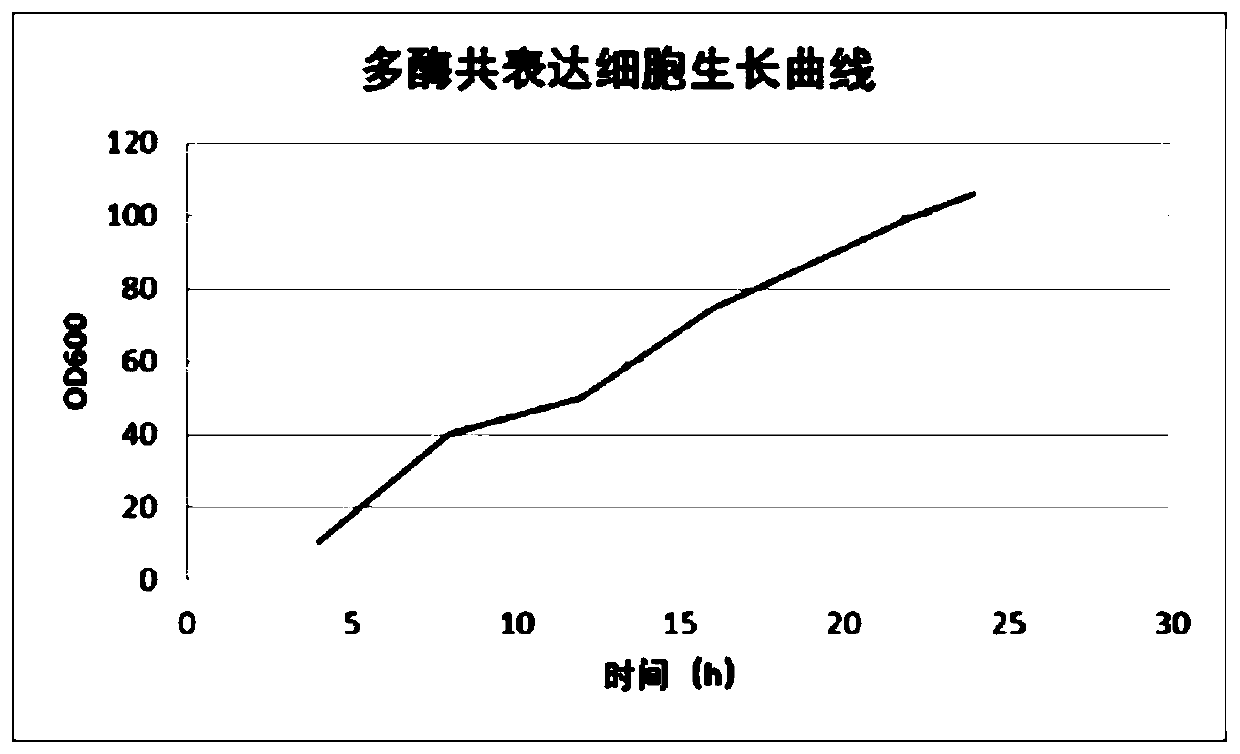
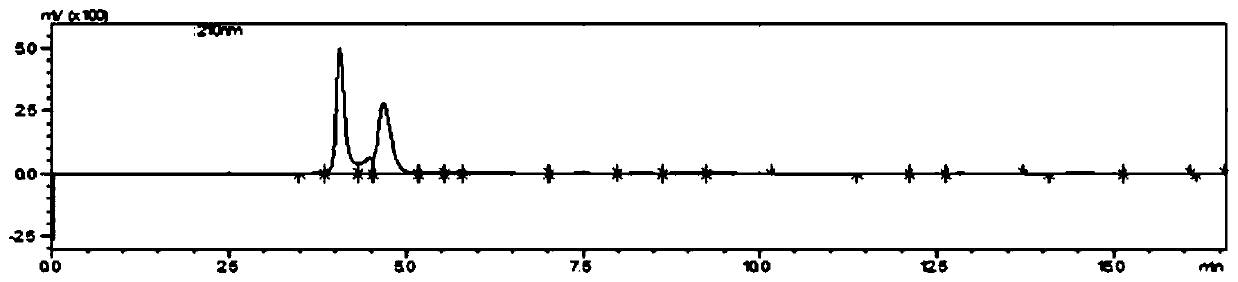
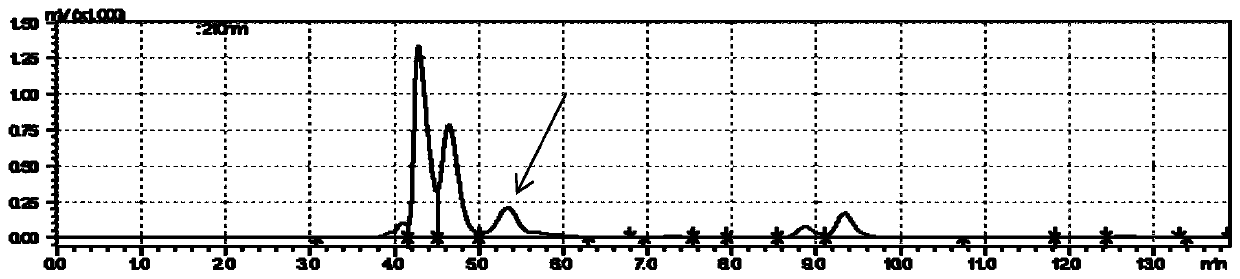
Method for preparing sialyllactose
A technology of sialyllactose and sialic acid, which is applied in the direction of fermentation, etc., can solve the problems of long fermentation period of sialyllactose, failure to meet the needs of industrialization, waste of fermentation and time cost, etc., to save fermentation and time cost, and transform system components Simple, short-cycle effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] The method for preparing sialyllactose provided in this embodiment comprises the following steps:
[0067] 1 Construction of a multi-enzyme co-expression system (1) Construct the CMP-sialic acid synthetase (CMP-NeuAc synthetase) gene (SEQ ID NO.1) on the pACYCDuet-1 vector MCS1 using the restriction endonuclease sites BamHI and HindIII (See Figure 10 ), forming the recombinant plasmid pACYCDuet-CMPNs.
[0068] (2) Design primers with NdeI and AvrII restriction sites so that the 3' end of the α-2,3-sialyltransferase gene (SEQ ID NO.2) is amplified with a 6×His histidine tag. The amplified product and the recombinant plasmid pACYCDuet-CMPNs were digested with endonucleases NdeI and AvrII respectively, and the α-2,3-sialyltransferase gene containing histidine tag was constructed into the plasmid under the action of T4 ligase On pACYCDuet-CMPNs MCS2, the recombinant plasmid pACYCDuet-CMPNs-ST was formed.
[0069] (3) Transfer the recombinant plasmid into BL21(DE3) compe...
Embodiment 2
[0085]After the reaction in Example 1 is over, use a magnet to absorb the magnetic nanoparticles and discard the reaction solution. After washing the immobilized enzyme twice with a buffer, continue to put it into the reaction kettle. The reaction raw materials are put in the same way as in Example 1. Phase analysis detected that 44.5g / L sialyllactose was obtained, and the conversion rate was 88%, which indicated that the co-immobilized enzyme realized the reuse of biocatalyst.
Embodiment 3
[0087] This example provides a method for preparing 6'-sialyllactose, which is basically the same as in Example 1, except that 6'-sialyllactose is prepared using α-2,6-sialyltransferase instead of α-2,3 - sialyltransferase. That is, the α-2,6-sialyltransferase gene (SEQ ID NO.5) is used to replace the α-2,3-sialyltransferase gene in Example 1, and other methods for preparing immobilized enzymes according to Example 1 And transformation conditions, after transformation 12, 6'-sialyllactose content is 46.6g / L in the system, and transformation rate is 92% ( Figure 13 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com