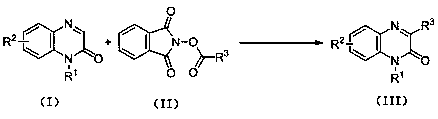
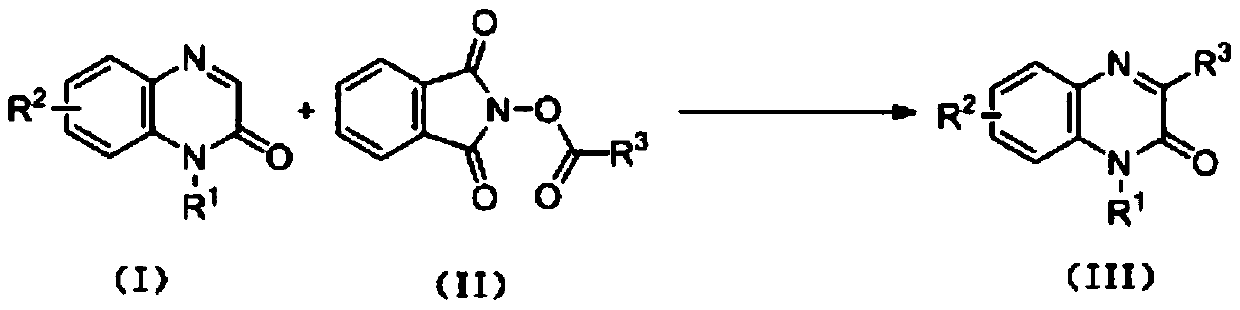
Synthetic method for C-3 position alkyl-substituted quinoxalinone derivatives based on Minisci reaction
A quinoxalinone and synthesis method technology, which is applied in the field of synthesis of C-3 alkyl substituted quinoxalinone derivatives, can solve the problems of increasing the emission of toxic and harmful substances, increasing energy consumption, high reaction temperature, etc., and achieving Reduce waste discharge, save resources, and have good reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add N-methylquinoxalinone (1.0mmol, 160mg), N-(2,2-dimethyl)propionyloxyphthalimide (1.2mmol, 296mg), trifluoroacetic acid (0.5mmol, 57mg), Na 2 -eosin Y (0.01mmol, 7mg), DMSO (3.0mL), reaction system with N 2 Protected and reacted at room temperature for 40h under 3W white light irradiation. After the reaction is finished, the reaction solution is washed with water and extracted with dichloromethane, and then separated to obtain an aqueous layer and an organic layer. The organic layer is dried with anhydrous sodium sulfate, and the filter residue is removed by filtration. Separation, using a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 15:1 as an eluent, collecting the eluate containing the target compound, evaporating the solvent and drying to obtain the target product N-methyl-3-tert-butyl Quinoxalinone (white solid) 209mg, yield is 97%, its chemical structural formula is:
[0030] Characterization data: white solid, melting point: 6...
Embodiment 2
[0032] The photocatalyst (Na 2 -eosin Y) is replaced by RoseBengal of equivalent molar amount, other operation is the same as embodiment 1, the quality that obtains target product N-methyl-3-tert-butylquinoxalinone (white solid) is 108mg, and its yield is 50 %.
Embodiment 3
[0034] Replace the white light source in the system with green light or blue light of the same power, other operations are the same as in Example 1, and the quality of the target product N-methyl-3-tert-butylquinoxalinone (white solid) is 162mg and 106mg, the yields were 75% and 49%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com