Polypeptide and application thereof
An amino acid and drug technology, applied in the field of peptides, can solve the problems of low safety, single pharmacological target, and high toxicity of chemical drugs, and achieve the effects of high safety, inhibition of proliferation and activation, and improvement of lung tissue structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
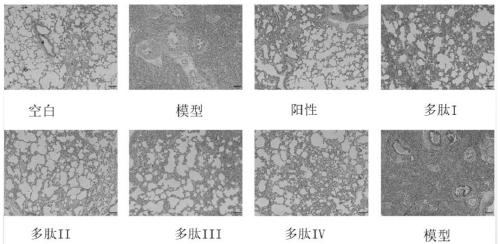
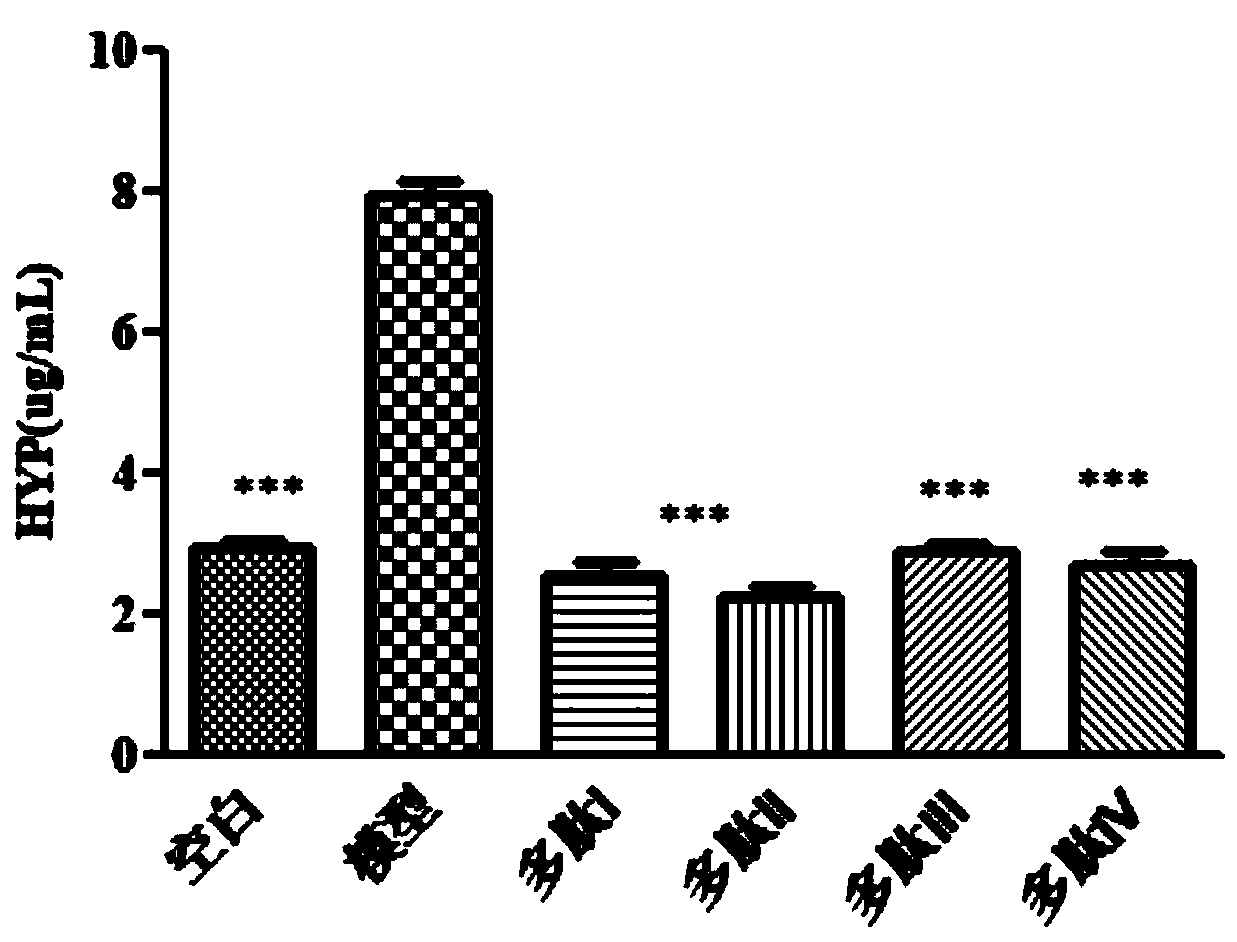
[0063] Embodiment 1, pulmonary fibrosis animal model
[0064] Experimental animals and materials:
[0065] 1. Experimental animals:
[0066] Source, Species, Strain: SPF grade, SD rats, purchased from Shanghai Sipro-Bikay Laboratory Animal Co., Ltd.
[0067] Provided (Experimental Animal License: SCXK (Shanghai) 2013-0016)
[0068] Weight: 180-200g at the time of purchase, 180-220g at the beginning of modeling
[0069] Gender: Male.
[0070] Number of animals in each group: 14 in each group.
[0071] 2. Experimental materials:
[0072]
[0073] 3. Experimental method:
[0074] SD rats were anesthetized by intraperitoneal injection of 1 mL / 100 g and 4% chloral hydrate. After the rats were anesthetized, the rats were fixed, and the necks of the rats were disinfected with 75% alcohol cotton. Cut the rat neck skin longitudinally with scissors, and bluntly tear the fascia and muscles longitudinally with forceps to expose the trachea. A syringe was inserted into the trach...
Embodiment 2
[0086] Example 2. Establishment of In Vitro Liver Fibrosis Model and Peptide Therapy
[0087] 1. Experimental materials:
[0088] Alkaline HYP Kit Manufacturer: Nanjing Jiancheng
[0089] 2. Experimental method:
[0090] Culture LX-2 cells with 1640 medium containing 10% FBS, and place it at 37°C, 5% CO 2 Cultured in an incubator, the medium was changed every 2 days, and the cells were passaged according to the density of the cells. Take the LX-2 cells in the logarithmic growth phase, digest them with 0.25% trypsin, make a cell suspension, and adjust the cell density to 4×10 5 cells / mL, inoculate them in 96-well plates, 100 μL per well, culture overnight, add TGF-β1 the next day to induce the proliferation and activation of LX-2 cells, and give peptide drugs 1 μmol / L at the same time, except for the blank group, no TGF-β1 was added. β1 and other groups were added with TGF-β1 (10ng / mL), cultured in the incubator for 48h, then added 10μL of MTT to each well, sucked out the M...
Embodiment 3
[0100] Embodiment 3, establishment of renal fibrosis model
[0101] 1. Experimental animals
[0102] Clean-grade male SD rats were purchased from Nanjing Qinglongshan Animal Farm, with a body weight of 180-200g when purchased, 190-210g when the model was started, and 180-200g when the drug was started.
[0103] 2. Experimental materials:
[0104] Normal saline Manufacturer: Anhui Double Crane Pharmaceutical Co., Ltd.
[0105] Rat TGF-β1 ELISA Kit Manufacturer: Tianjin Anoricang Biotechnology Co., Ltd.
[0106] Alkaline HYP Kit Manufacturer: Nanjing Jiancheng
[0107] 3. Experimental method
[0108] To establish an animal model of renal fibrosis, SD rats were anesthetized with 4% chloral hydrate, intraperitoneally injected with 1mL / 100g, fixed on the operating board, and the operating area was sterilized for later use, about 3- Cut open the abdominal cavity at 4 mm, and the surgical team treated the left kidney and ureter, ligated and separated the ureter near the lower po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com