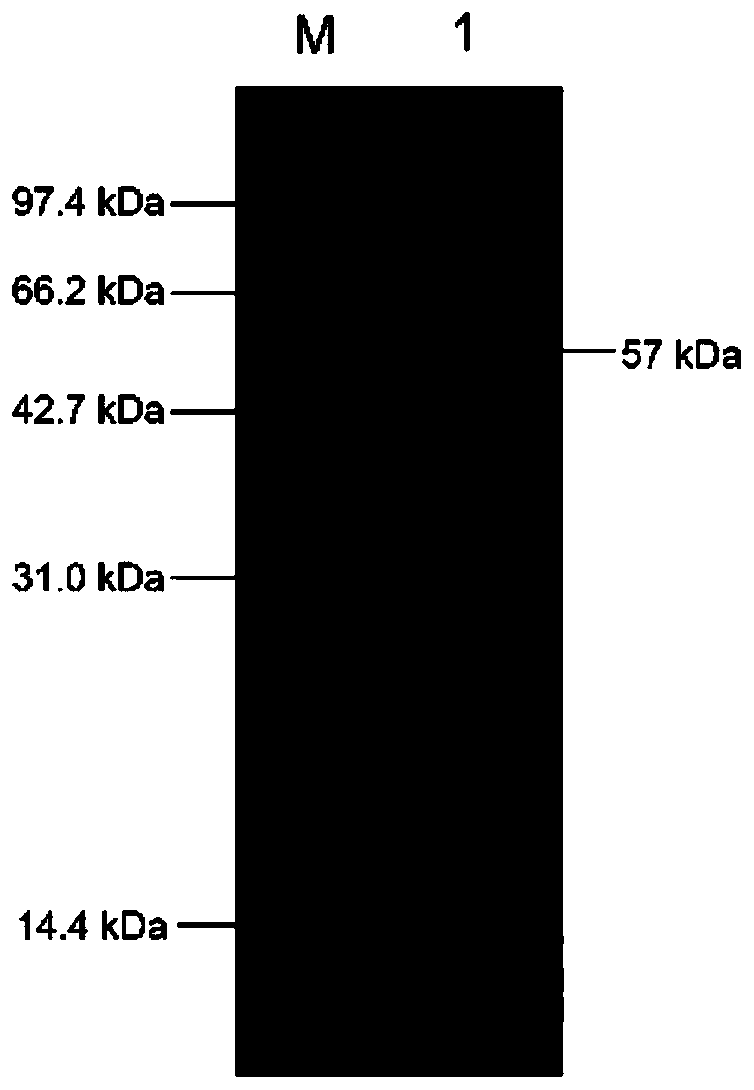
Alginate lyase and application thereof
A technology of alginate lyase and algae oligosaccharide, which is applied in the directions of lyase, application, biochemical equipment and method, can solve the problems of high price, few types of enzyme preparations, restriction enzyme application and development, etc., and achieves high product specificity. , stable nature, important industrial application value and scientific research value effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The production method of embodiment 1 alginate lyase Aly01
[0021] A Screening Method for Vibrio natriegens
[0022] (1) The sea mud was sampled near the kelp breeding factory in Rongcheng City, Shandong Province, and 1g of the sample was taken and dispersed evenly in 50mL of sterile water.
[0023] (2) Inoculate 1 mL of supernatant into 50 mL of screening liquid medium, culture at 28°C and 200 rpm for 2 days, dilute 10 -6 And spread it on the screening plate medium, culture at 28°C for 2 days, pick single colonies of different shapes and streak the plate several times to obtain pure culture.
[0024] (3) Pick single colonies of different shapes, inoculate them in liquid medium, and culture them at 28°C and 200rpm for 2 days, take the supernatant to measure the activity of alginate lyase, select a strain with higher enzyme activity, and entrust the Chinese typical culture The morphological characteristics, physiological and biochemical characteristics and 16S rDNA se...
Embodiment 2
[0039] Example 2 Sequence Alignment
[0040] After amino acid sequencing of the enzyme, primers were designed to amplify the gene encoding alginate lyase from the genome of Vibrio natriegens SK42.001, the nucleotide sequence of which is shown in SEQ ID NO:2. DNA sequence BLAST results: The alginate lyase provided by the present invention has the closest DNA sequence homology to the alginate lyase derived from Vibrio alginolyticus FDAARGOS, but the similarity is only 85%, with 231 base differences and 8 gaps.
[0041] Amino acid sequence BLAST results: the alginate lyase provided by the present invention has the closest amino acid sequence homology with an alginate lyase derived from the genus Vibrio in the NCBI database, with a similarity of 93%, 39 amino acid differences, and 0 gap.
Embodiment 3
[0042] Embodiment 3 enzymatic properties
[0043] Enzyme activity definition: the amount of enzyme required to produce 1 μmol of reducing sugar per minute.
[0044] Enzyme activity detection method: 1mL enzyme reaction solution (50mM, pH7.0 PB buffer solution) contains: 5mg sodium alginate, 300mM NaCl, 0.84μg alginate lyase (enzyme purified in Example 1), react at 35°C for 30min, The fermentation supernatant was taken to detect the enzyme activity by DNS method.
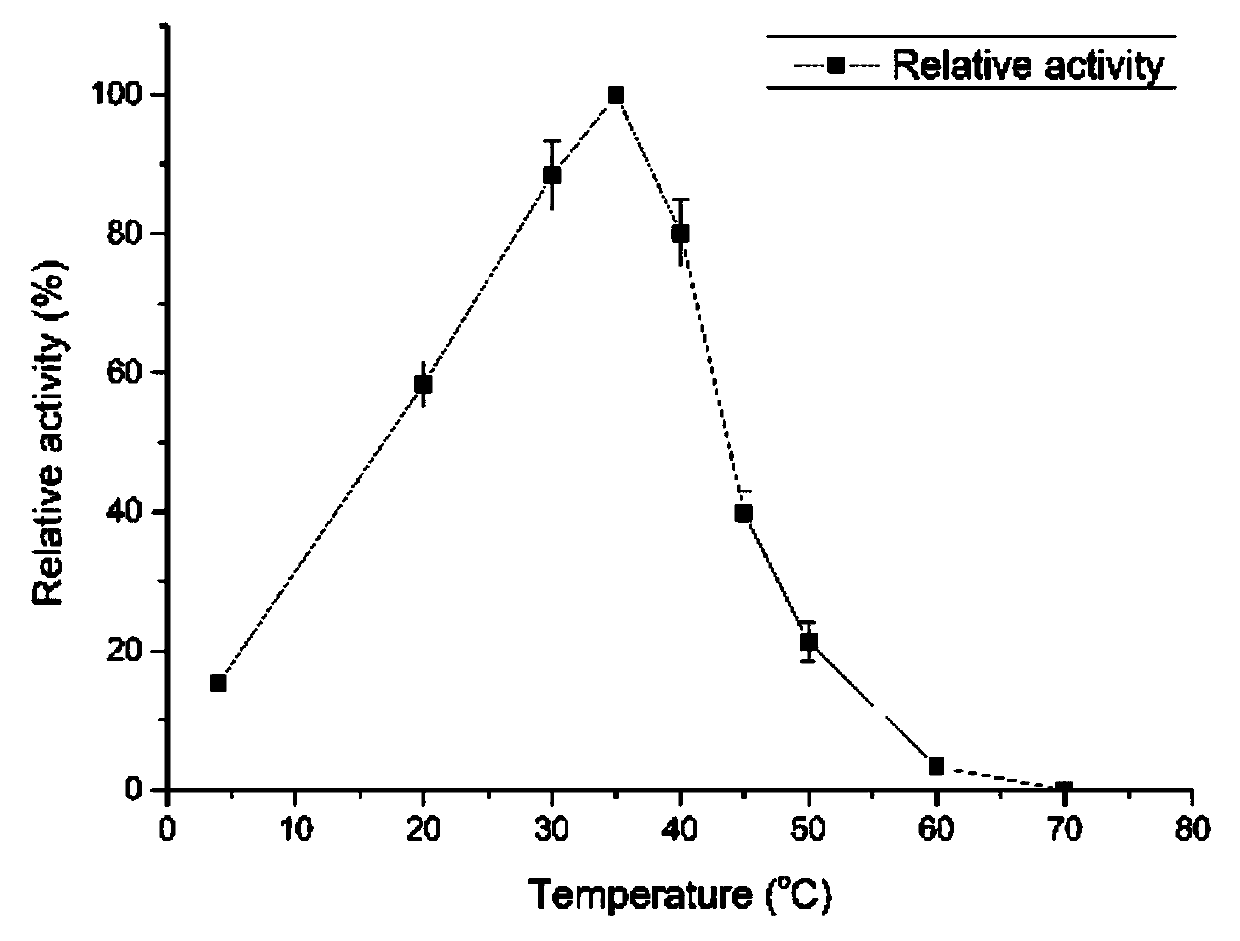
[0045] (1) Effect of temperature on enzyme activity: 1mL enzyme reaction solution (50mM, PB buffer solution with pH7.0) contains: 5mg sodium alginate, 300mM NaCl, 0.84μg alginate lyase; place the enzyme reaction solution in 4 °C, 20 °C, 30 °C, 35 °C, 40 °C, 50 °C, 60 °C and 70 °C in a water bath for 30 min, and measure the activity of alginate lyase at each temperature. Such as figure 2 As shown, the optimum reaction temperature is 35°C.
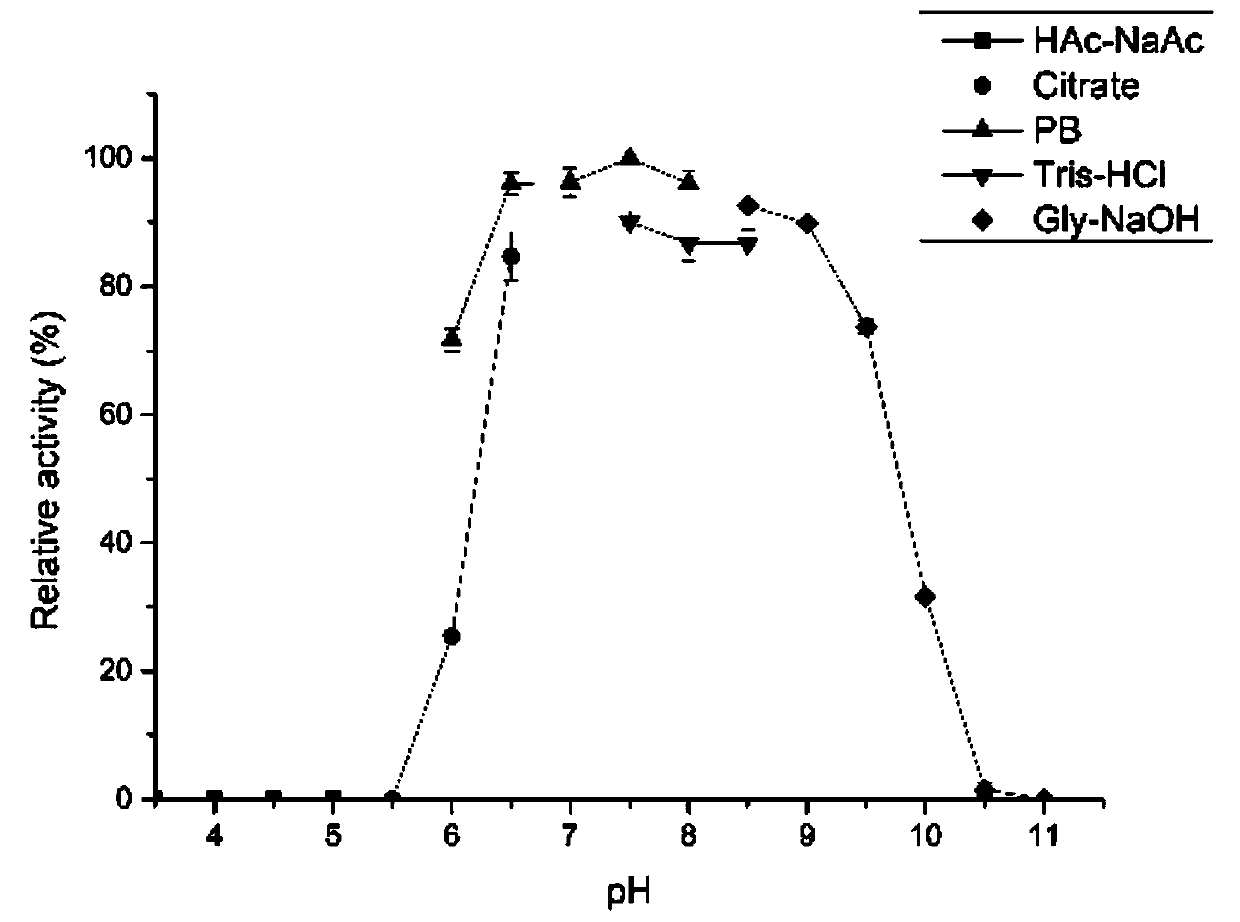
[0046] (2) Effect of pH on enzyme activity: 1mL enzyme reaction solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com