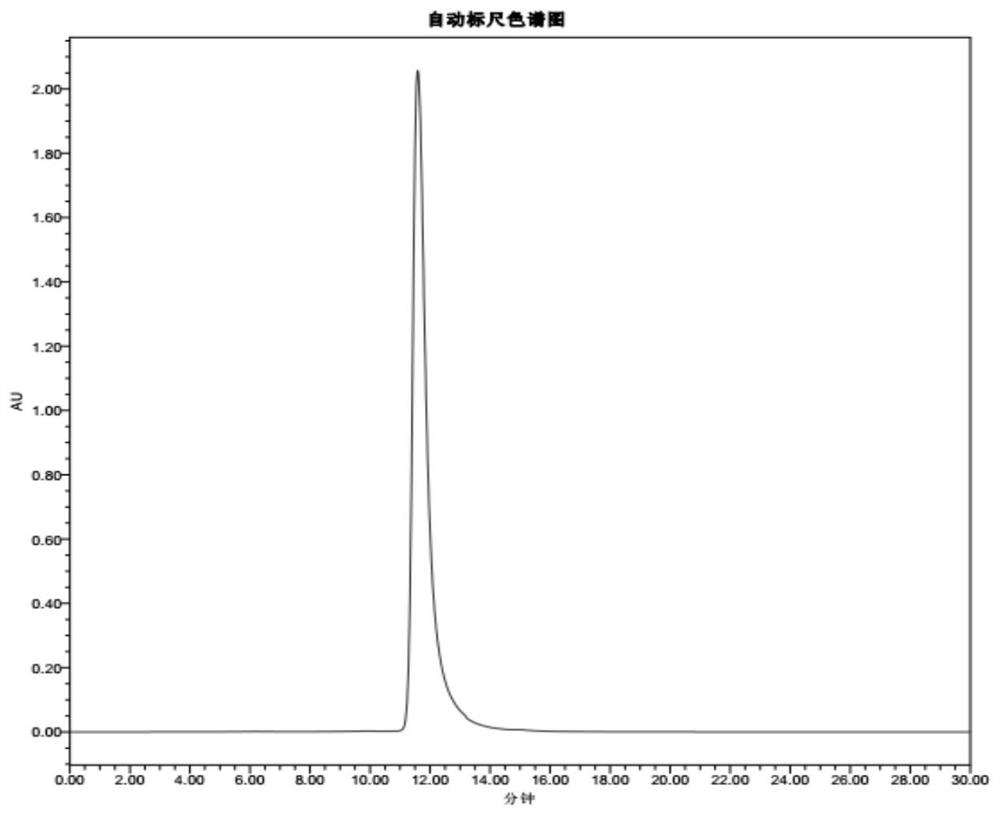
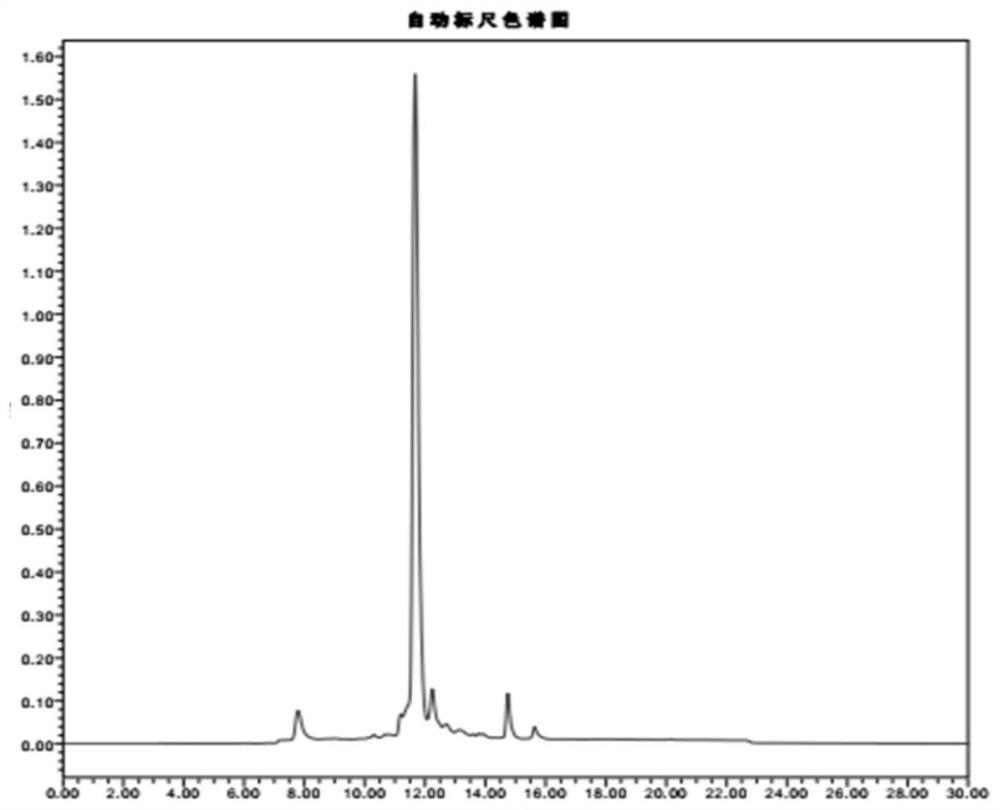
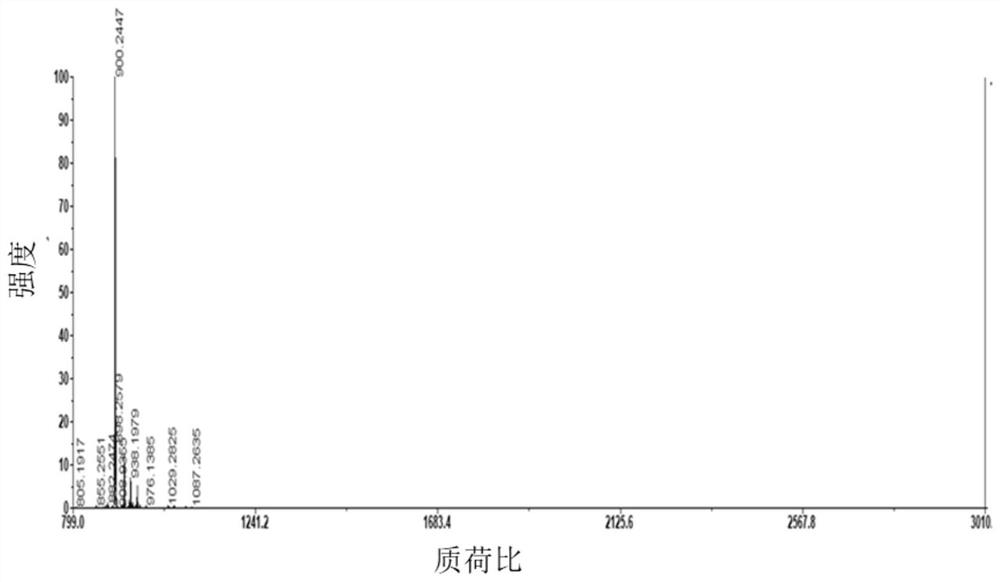
A preparation method of double-enzyme co-immobilized copper nanoflower material and its application in glucose detection
A copper nanometer and nanoflower technology, applied in the field of glucose detection, can solve the problems of affecting sensitivity, time-consuming and cumbersome operations, restricting the development of glucose detection, etc., and achieve the effects of improving efficiency, sensitivity and high peroxidase activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Solid phase synthesis of Dh-β-Ala-His-Glu
[0041] (1) Resin swelling: weigh 0.1mmol Rink-NH 2 Resin, add dichloromethane reagent to fully infiltrate the resin, swell for 1 hour, remove the dichloromethane by suction filtration, and wash the resin 6 times with dimethylformamide (DMF).
[0042] (2) Deprotection: to step (1) processed Rink-NH 2 Add DEP (DMF containing 20% piperidine) solution to the resin, shake at room temperature for 15-30min, filter with suction, wash the resin 6 times with DMF, and use the color developer (A: 5% ninhydrin ethanol solution B: 80% Phenol ethanol solution) to detect the deprotection of the amino group, when the resin particles all turn blue-purple, the deprotection is complete.
[0043] (3) Linking amino acids: Fmoc-Glu(otBu)-OH, PyBOP (benzotriazol-1-yl-oxytripyrrolidinylphosphonium hexafluorophosphate), HOBT (1-Hydroxybenzotriazole), NMM (N- methylmorpholine) and Rink-NH 2 The resin is added to the reactor sequentially a...
Embodiment 2
[0050] Embodiment 2: The solid-phase synthesis of Dh-β-Ala-His-Lys
[0051] (1) Repeat step (1) of Example 1.
[0052] (2) Repeat step (2) of Example 1.
[0053] (3) Connecting amino acids: Fmoc-Lys(Boc)-OH, PyBOP (benzotriazol-1-yl-oxytripyrrolidinylphosphonium hexafluorophosphate), HOBT (1-Hydroxybenzotriazole), NMM (N- methylmorpholine) and Rink-NH 2 The resin is added to the reactor sequentially at a molar ratio of 3:3:3:6:1, and about 2 / 3 of the container volume of dimethylformamide is added to shake at room temperature for 1.5-2 hours, filtered with suction, and the resin is washed with dimethylformamide 6 times, use a chromogenic reagent to detect the amino acid connection, and the resin particles all become colorless, indicating that the reaction is complete.
[0054] (4) Repeat step (4) of Example 1.
[0055] (5) Repeat step (5) of Example 1.
[0056] (6) Repeat step (6) of Example 1.
[0057] (7) Repeat step (7) of Example 1.
[0058] (8) Repeat step (8) of Ex...
Embodiment 3
[0060] Example 3: Solid phase synthesis of Dh-β-Ala-His-Asp.
[0061] (1) Repeat step (1) of Example 1.
[0062] (2) Repeat step (2) of Example 1.
[0063] (3) Connecting amino acids: Fmoc-Asp(OtBu)-OH, PyBOP (benzotriazol-1-yl-oxytripyrrolidinylphosphonium hexafluorophosphate), HOBT(1-Hydroxybenzotriazole), NMM(N- methylmorpholine) and Rink-NH 2 The resin is added to the reactor sequentially at a molar ratio of 3:3:3:6:1, and about 2 / 3 of the container volume of dimethylformamide is added to shake at room temperature for 1.5-2 hours, filtered with suction, and the resin is washed with dimethylformamide 6 times, use a chromogenic reagent to detect the amino acid connection, and the resin particles all become colorless, indicating that the reaction is complete.
[0064] (4) Repeat step (4) of Example 1.
[0065] (5) Repeat step (5) of Example 1.
[0066] (6) Repeat step (6) of Example 1.
[0067] (7) Repeat step (7) of Example 1.
[0068] (8) Repeat step (8) of Example 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com