Electrochemical preparation method of alkynyl thiocyanate
An alkynyl thiocyanate, electrochemical technology, applied in the electrolysis process, electrolysis components, electrolysis organic production and other directions, can solve the problems of complex reaction raw materials, and achieve the effects of simple and easy-to-obtain raw materials, low commercial price, and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
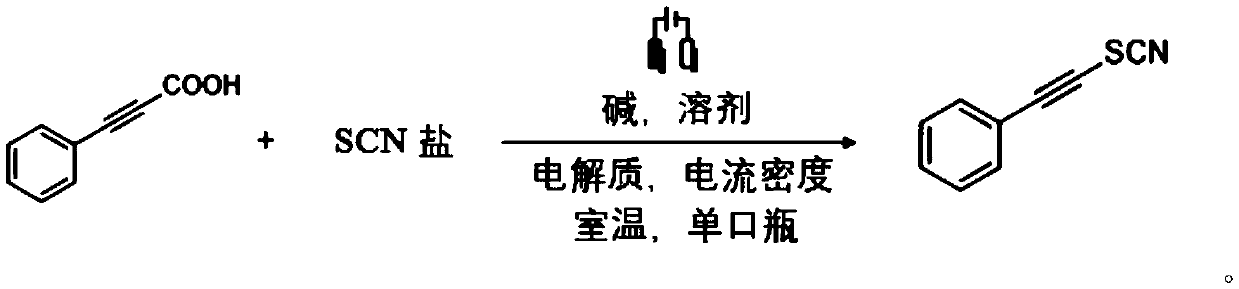
[0036] With the platinum sheet as the anode and the platinum sheet as the cathode, add 0.2mmol phenylpropylic acid, 0.26mmol ammonium thiocyanate, 0.3mmol ammonium acetate, 0.4mmol ammonium formate, 0.1M potassium perchlorate, 6ml solvent ( Acetonitrile: water = 9:1), magnetic stirrer, turn on the power, adjust the current to 8mA, and react at room temperature for 6h. After the reaction, it was extracted three times with ethyl acetate, the organic phases were combined, dried with anhydrous magnesium sulfate, nitromethane was added as an internal standard, the yield was 82%, and the corresponding product was obtained after separation, rotary evaporation in vacuum, and purification.
[0037] The reaction of the present embodiment is shown in the following formula:
[0038]
[0039] Product NMR data:
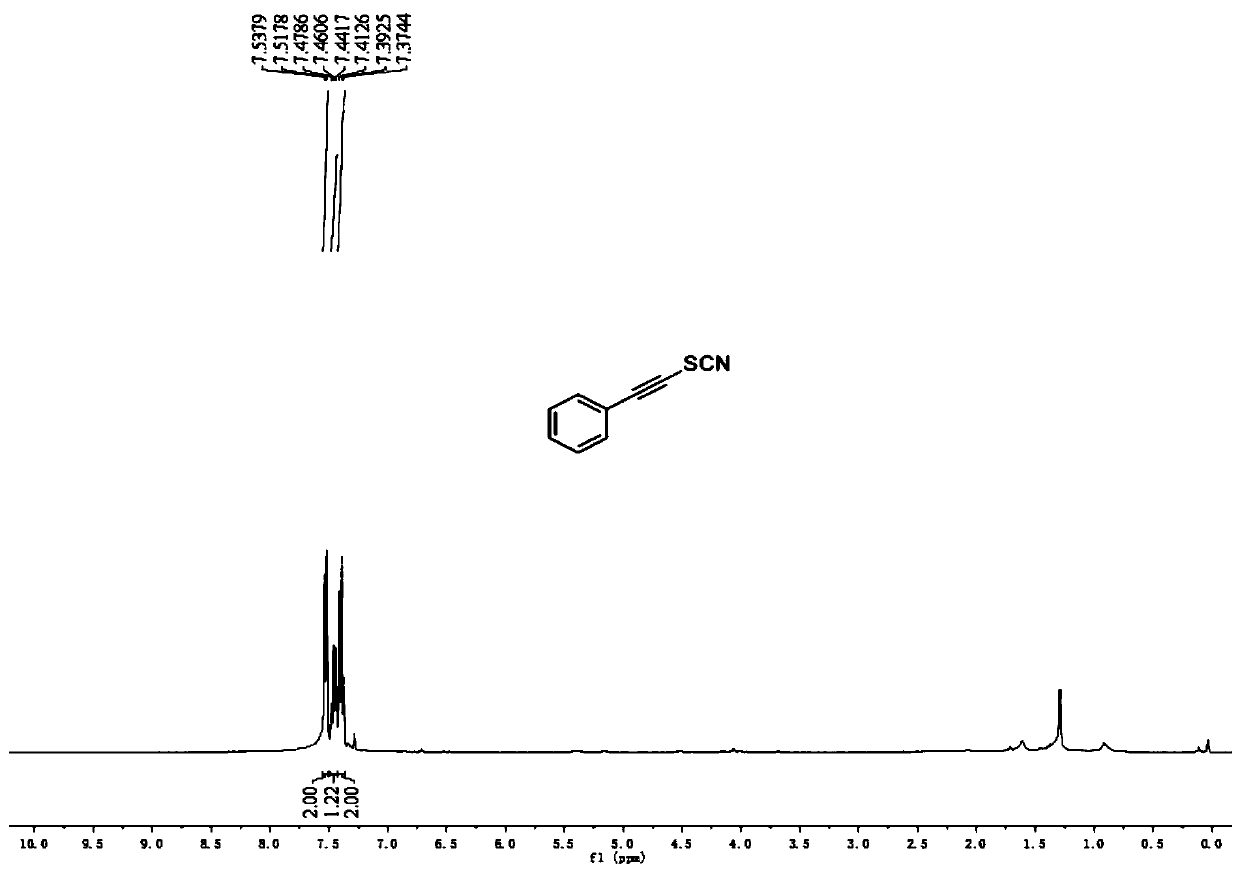
[0040] 1 H NMR (400MHz, CDCl 3 ):δ7.51-7.49(m,2H),7.48-7.42(m,1H),7.37-7.35(m,2H)ppm.
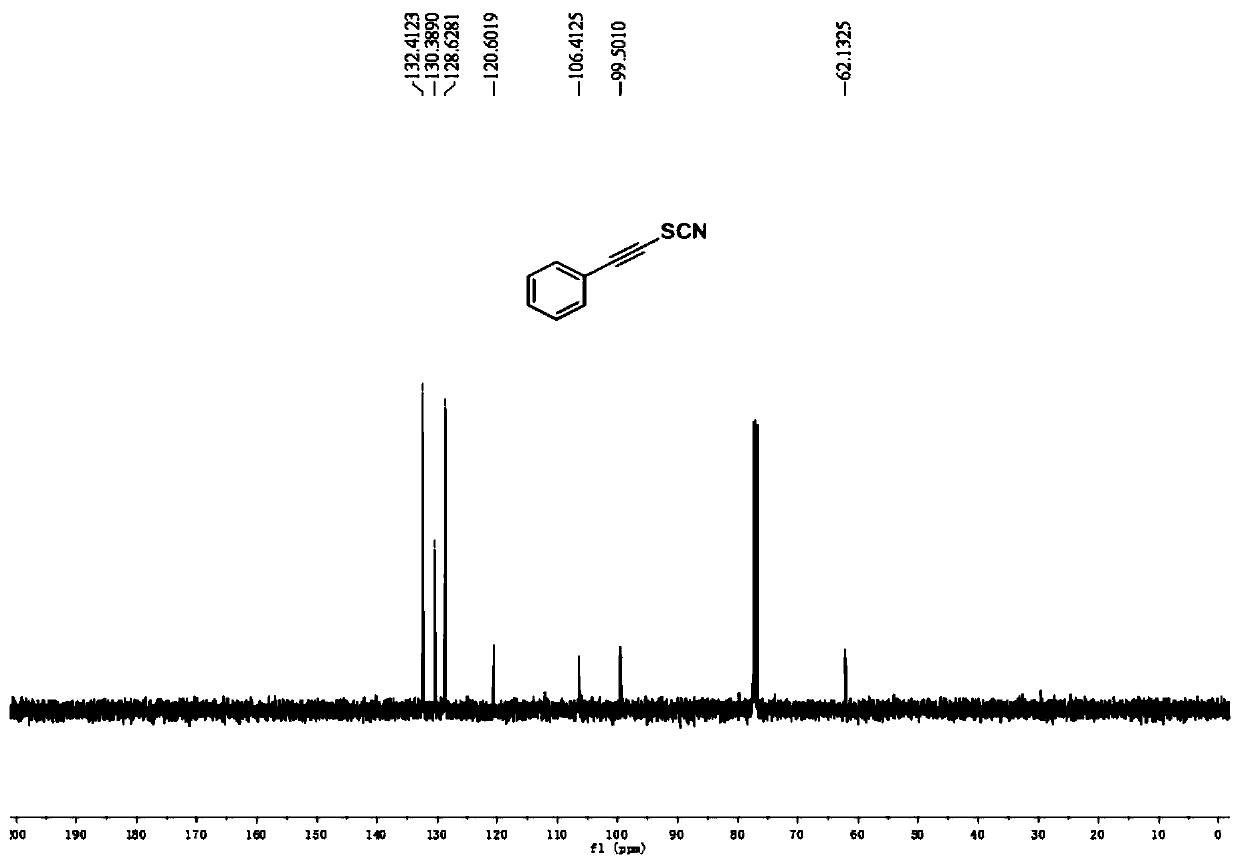
[0041] 13 C NMR (100MHz, CDCl 3 ): δ132.4, 130.4, 128.6, 120.6, 106.4, 99.5, 62.1. ...
Embodiment 2
[0045] With the platinum sheet as the anode and the platinum sheet as the cathode, add 0.2mmol phenylpropylic acid, 0.26mmol ammonium thiocyanate, 0.3mmol ammonium acetate, 0.6mmol ammonium formate, 0.1M potassium perchlorate, 6ml solvent ( Acetonitrile: water = 9:1), magnetic stirrer, turn on the power, adjust the current to 8mA, and react at room temperature for 6h. After the reaction, it was extracted three times with ethyl acetate, the organic phases were combined, dried with anhydrous magnesium sulfate, nitromethane was added as an internal standard, the yield was 77%, and the corresponding product was obtained after separation, vacuum rotary evaporation, and purification.
[0046] The reaction of the present embodiment is shown in the following formula:
[0047]
[0048] Product NMR data:
[0049] 1 H NMR (400MHz, CDCl 3 ):δ7.51-7.49(m,2H),7.48-7.42(m,1H),7.37-7.35(m,2H)ppm.
[0050] 13 C NMR (100MHz, CDCl 3 ): δ132.4, 130.4, 128.6, 120.6, 106.4, 99.5, 62.1. of ...
Embodiment 3
[0054] With the platinum sheet as the anode and the platinum sheet as the cathode, add 0.2mmol phenylpropylic acid, 0.26mmol ammonium thiocyanate, 0.3mmol ammonium acetate, 0.2mmol ammonium formate, 0.1M potassium perchlorate, 6ml solvent ( Acetonitrile: water = 9:1), magnetic stirrer, turn on the power, adjust the current to 8mA, and react at room temperature for 6h. After the reaction, it was extracted three times with ethyl acetate, the organic phases were combined, dried with anhydrous magnesium sulfate, nitromethane was added as an internal standard, the yield was 71%, and the corresponding product was obtained after separation, rotary evaporation in vacuum, and purification.
[0055] The reaction of the present embodiment is shown in the following formula:
[0056]
[0057] Product NMR data:
[0058] 1 H NMR (400MHz, CDCl 3 ):δ7.51-7.49(m,2H),7.48-7.42(m,1H),7.37-7.35(m,2H)ppm.
[0059] 13 C NMR (100MHz, CDCl 3 ): δ132.4, 130.4, 128.6, 120.6, 106.4, 99.5, 62.1. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com