Aprepitant nanocrystal suspension and preparation method and application thereof
A nano-crystal, aprepitant technology, applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve the problem of poor stability of aprepitant nano-crystal liquid and harsh storage conditions. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
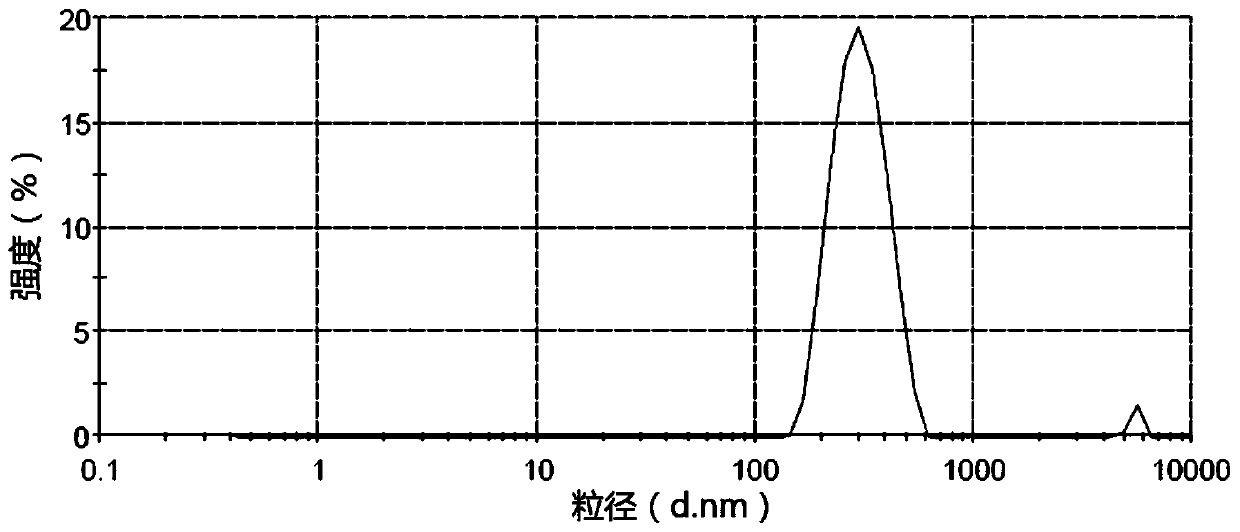
Embodiment 1
[0135] prescription:
[0136] Aprepitant 200mg Povidone K60 200mg Sodium dodecyl sulfate 50mg Croscarmellose Sodium 2.0g pregelatinized starch 4.0g sodium benzoate 0.2g sucrose 50g strawberry flavor 2mL
[0137] Preparation process: mix the prescribed amount of aprepitant with povidone K60 and sodium lauryl sulfate, add an appropriate amount of deionized water, and place it in a high-pressure homogenizer for 10 times (homogeneous pressure 1000bar) , to get aprepitant nano crystallization liquid (A liquid). The croscarmellose sodium solution (add boiling water to make the concentration 40mg / mL), pregelatinized starch slurry (add boiling water to make the concentration 80mg / mL), syrup (sucrose is made according to the pharmaceutically acceptable process to make 75% concentration of syrup), sodium benzoate and strawberry essence were placed in a stirring device and stirred evenly to obtain a blank suspension (liquid B)...
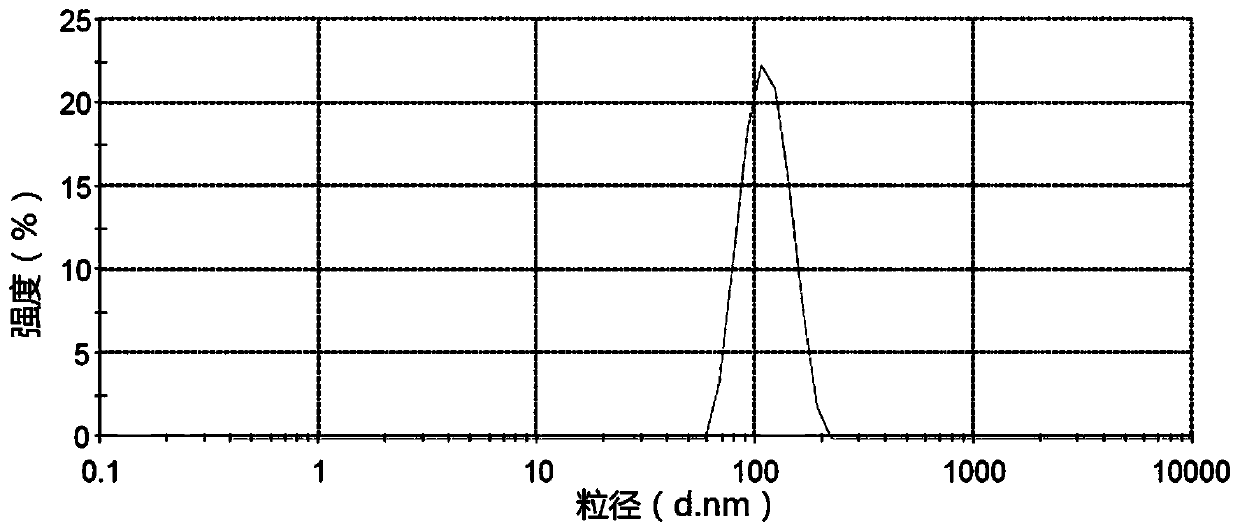
Embodiment 2
[0139] prescription:
[0140]
[0141]
[0142] Preparation process: After mixing the prescribed amount of aprepitant and methylcellulose solution (concentration: 1mg / mL), add an appropriate amount of deionized water, and place it in an experimental ball mill. After the zirconia beads were ground for 4 hours (grinding speed 500rpm), the aprepitant nanocrystal liquid (liquid A) was obtained. Corn starch slurry (add boiling water to make up to 80mg / mL), syrup (sucrose is made into a syrup with a concentration of 75% according to a pharmaceutically acceptable process), xanthan gum, poloxamer, methyl paraben, paraben Propyl ester, citric acid and orange essence were placed in a stirring device and stirred evenly to obtain a blank suspension (liquid B). Add liquid A to liquid B, mix evenly, add deionized water to make up the volume to 400mL, place it in a colloid mill and grind for 10 minutes to obtain the final oral suspension. The average particle diameter of the obtained...
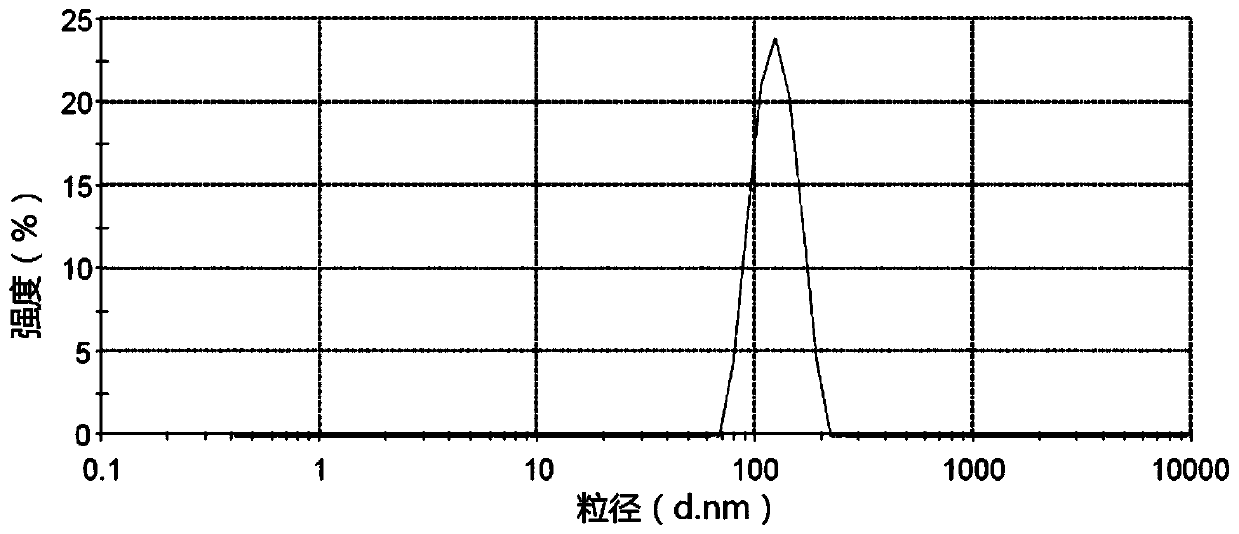
Embodiment 3
[0144] prescription:
[0145]
[0146]
[0147] Preparation process: After mixing the prescribed amount of aprepitant, povidone K30 and methylcellulose solution (concentration is 1mg / mL), after adding an appropriate amount of deionized water, homogenize 10 times in a high-pressure homogenizer ( Homogeneous pressure 1000bar), get aprepitant nano-crystallization liquid (A liquid). Hypromellose solution (add boiling water to make a concentration of 20 mg / mL), carboxymethylcellulose sodium solution (add boiling water to make a concentration of 20 mg / mL), syrup (sucrose is prepared according to a pharmaceutically acceptable process for 75 % concentration of syrup), xanthan gum, methylparaben, sodium benzoate, disodium hydrogen phosphate, mannitol and strawberry essence were placed in a stirring device and stirred evenly to obtain a blank suspension (liquid B). Add liquid A to liquid B, mix evenly, add deionized water to make up the volume to 400mL, place it in a colloid mill...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com