A bipyridine bisphenol-aluminum catalyst for preparing unsaturated polyester and its preparation method
A technology of bipyridyl bisphenol and catalyst, which is applied in the field of bipyridine bisphenol-aluminum catalyst for the preparation of unsaturated polyester and can solve the problems of lack of high activity and low biological toxicity metal organic complex catalytic system, etc. Achieve the effect of simple and efficient synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1-9
[0093] Specific examples 1-9 are at first the synthetic steps of ligands LA~LI:
[0094]
Embodiment 1
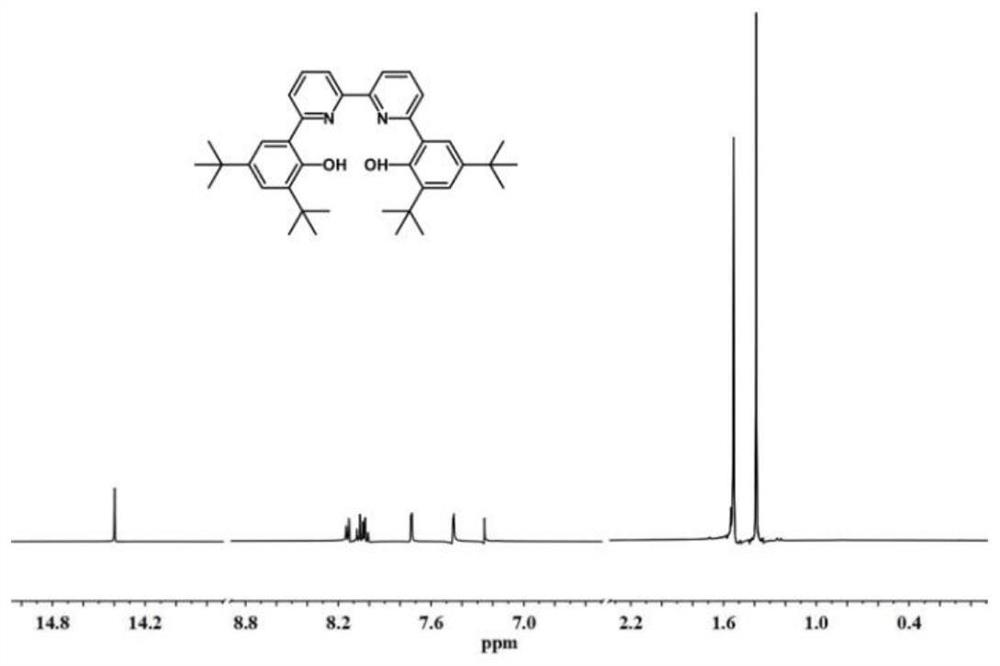
[0096] The synthesis steps of the compound LA: under the protection of nitrogen, dissolve 2,4-di-tert-butylphenol (10.3g, 50mmol) in 100mL of dichloromethane, and slowly add 55mL of liquid bromine dichloromethane at 0°C methane solution (1.0mol / L), gradually return to room temperature and react for 12h, and use NaHSO 3 The reaction was quenched, and the organic phase was extracted with dichloromethane, anhydrous NaSO 4 Dry, concentrate, and separate by column chromatography to obtain 13.49 g of 2,4-di-tert-butyl-6-bromophenol, with a yield of 95%. Under nitrogen, dissolve 2,4-di-tert-butyl-6-bromophenol in 1,4-dioxane, add [1,1'-bis(diphenylphosphino)ferrocene] di 3.5g of palladium chloride, 18.0g of biboronic acid pinacol ester and 14g of potassium acetate were reacted at 80°C for 16h. After the reaction was completed, the reaction solution was directly concentrated and separated by column chromatography to obtain a white solid 2,4-di-tert-butyl -6-Hydroxyphenylboronic acid...
Embodiment 2
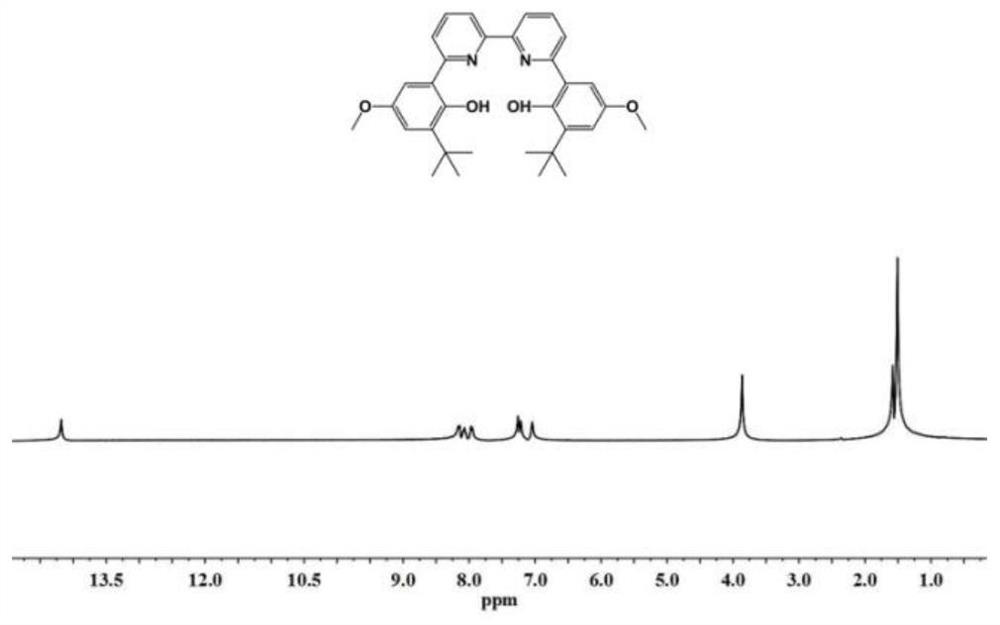
[0098] The synthesis steps of the compound LB: under the protection of nitrogen, dissolve 2-tert-butyl-4-methoxyphenol (9.0g, 50mmol) in 100mL of dichloromethane, slowly add 55mL of liquid bromine at 0°C dichloromethane solution (1.0mol / L), gradually return to room temperature and react for 12h, and use NaHSO 3 The reaction was quenched, and the organic phase was extracted with dichloromethane, anhydrous NaSO 4 Dry, concentrate, and separate by column chromatography to obtain 11.6 g of 2-tert-butyl-4-methoxy-6-bromophenol, with a yield of 90%. Under nitrogen, dissolve 2-tert-butyl-4-methoxy-6-bromophenol in 1,4-dioxane, add [1,1'-bis(diphenylphosphino)diocene Iron] 3.0g of palladium dichloride, 17.1g of biboronic acid pinacol ester and 13.5g of potassium acetate were reacted for 16h at 80°C. After the reaction was completed, the reaction solution was directly concentrated and separated by column chromatography to obtain a white solid 2-tert-butyl 7.3 g of 4-methoxy-6-hydroxy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com