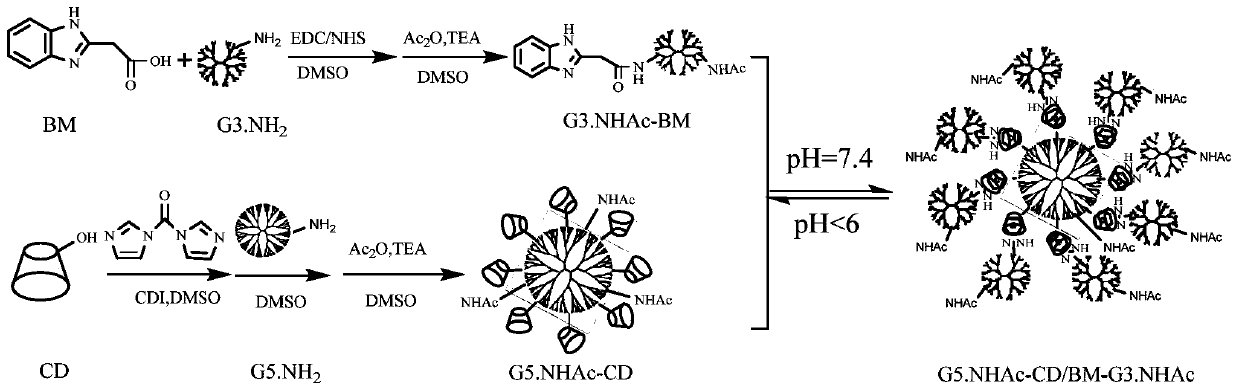
Preparation method of pH-responsive core-shell structure dendrimer drug carrier
A dendrimer and amine dendrimer technology, which is applied in the field of preparation of core-shell structure dendrimer drug carriers, can solve problems such as improving release efficiency, and achieves reduction of toxic and side effects, simple reaction conditions, and high drug The effect of loading rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Weigh 1.97 mg of benzimidazole-2-acetic acid (BM-COOH) and dissolve it in 5 mL of DMSO. Weigh 32.21mg EDC and 19.34mg NHS, dissolve them in 5mL DMSO respectively, first drop the EDC solution dissolved in DMSO into the BM-COOH solution and stir at room temperature for 30min, then add the NHS solution dissolved in DMSO dropwise into the reaction solution Stir continuously for 3h, BM-COOH can be activated. Weigh 50 mg of the third-generation polyamidoamine dendrimer (PAMAM G3) and dissolve it in 10 mL of DMSO. Then the above activated BM-COOH solution was added dropwise into the vigorously stirred G3 solution, and the reaction was continued under stirring for 3 days at room temperature. After the reaction was completed, the reaction solution was transferred to a dialysis bag with a molecular weight cut-off of 1000 Da, dialyzed in PBS buffer for 1 day, and then replaced with ultrapure water for dialysis for 2 days. Finally, the powder product G3.NH was obtained by freeze-...
Embodiment 2
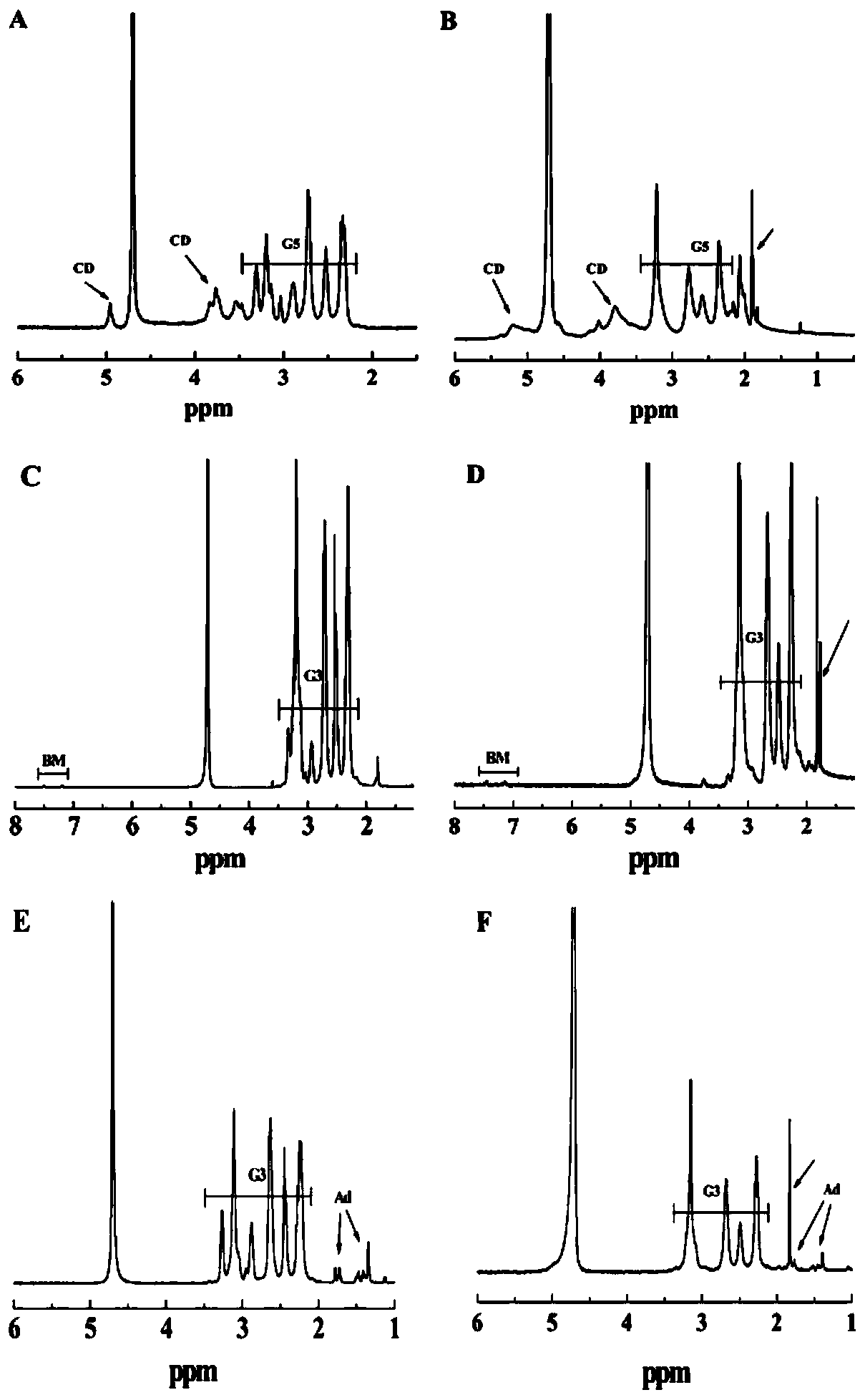
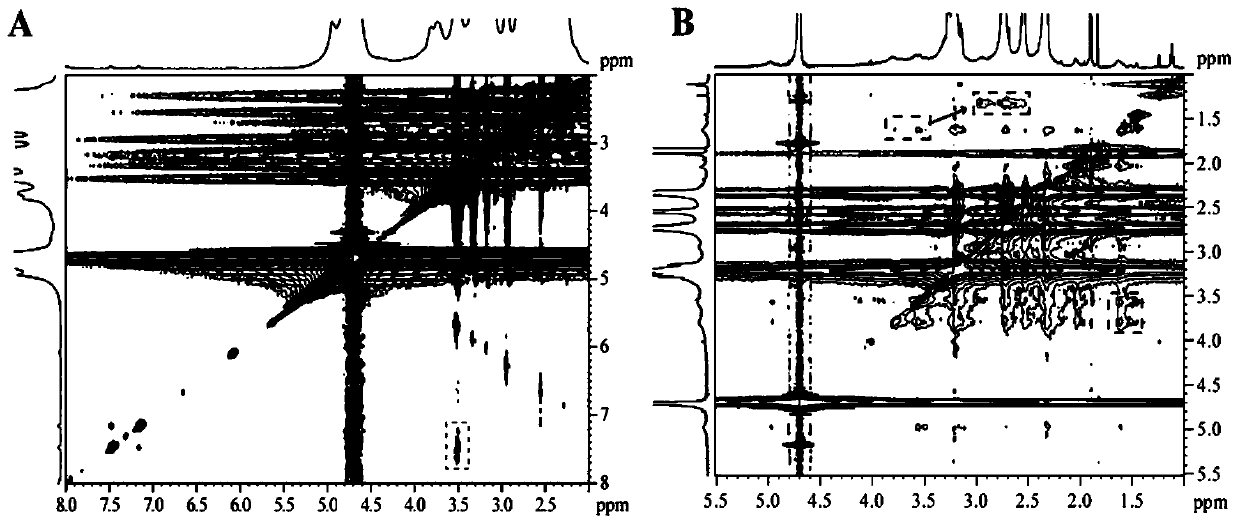
[0048] The dendrimers prepared in Example 1 were characterized. G5.NH 2 -CD's 1 H NMR characterization results as figure 2 As shown in A, the proton peak at chemical shift 2.25-3.4ppm represents the methylene proton peak of G5, and the proton peak at chemical shift 3.5-4.1ppm and 5.1ppm represents the proton peak in the molecular structure of β-CD, indicating that β-CD has been successfully modified on the surface of G5. By integrating the proton peak areas of G5 and β-CD, it was calculated that 8.2 β-CD molecules were surface-modified per G5. G3.NH 2 -BM's 1 HNMR characterization results such as figure 2 Shown in C, the proton peak at the chemical shift 7-8ppm represents the proton peak in the molecular structure of the benzimidazole-2-acetic acid group, and the proton peak at the chemical shift 2.2-3.4ppm represents the methylene proton peak of G3, It shows that BM-COOH has been successfully modified on the surface of G3. By integrating the proton peaks in these tw...
Embodiment 3
[0051] The materials G5.NHAc-CD / BM-G3.NHAc and G5.NHAc-CD / Ad-G3.NHAc prepared in Example 1 and Comparative Example 1 were weighed and dissolved in ultrapure water, then doxorubicin hydrochloride ( DOX·HCl) was dissolved in methanol solution, and then triethylamine was added to the DOX hydrochloride solution to completely dissolve DOX·HCl, and then the dissolved DOX was added drop by drop to the vigorously stirred shell-core structure tree In the aqueous solution of macromolecules, keep the pH of the whole system at about 7.4. The reaction was carried out at room temperature and protected from light, the bottle was opened and kept stirring for 24 hours, centrifuged at 7500r for 10 minutes, the supernatant was collected, and freeze-dried to obtain a core-shell structure dendrimer wrapped with DOX. Calculate the percentage of DOX uploaded onto the core-shell structured dendrimer from the pellet collected by centrifugation, and the drug loading amount of G5.NHAc-CD / BM-G3.NHAc and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com