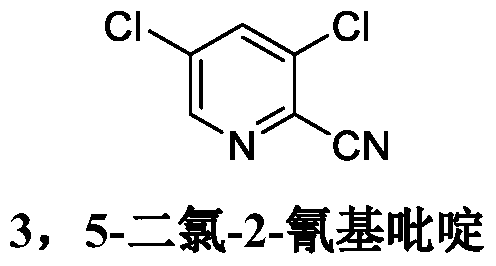
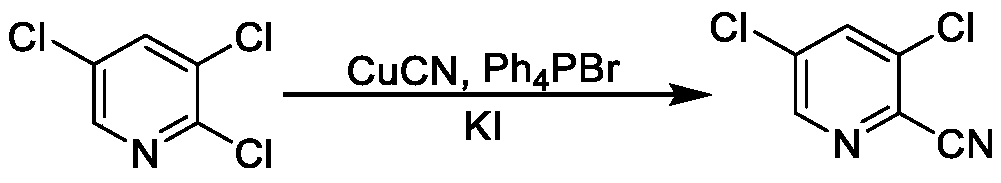
Synthesis method of 3, 5-dichloro-2-cyanopyridine
A synthetic method, the technology of cyanopyridine, applied in the chemical field, can solve the problems of high production cost and large dosage, and achieve the effects of reducing the generation of waste water, high yield and high content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
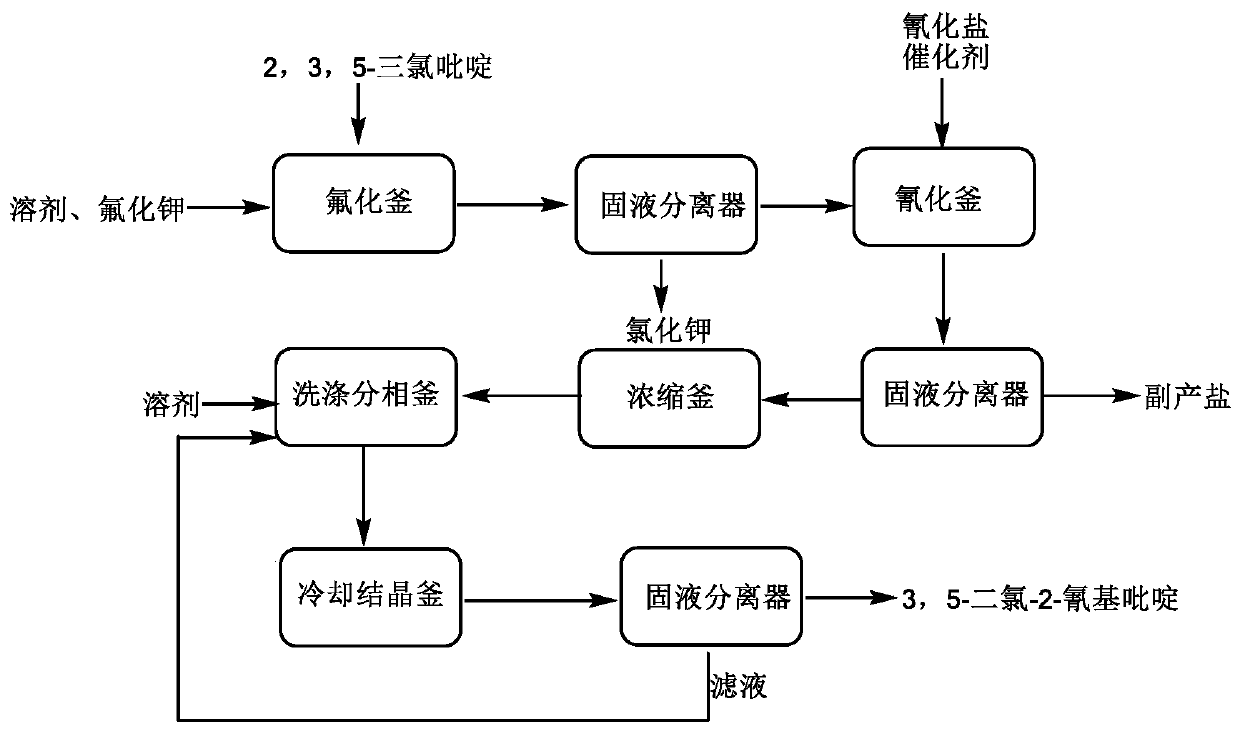
[0042] The synthetic method of the present embodiment comprises the steps:
[0043] (1) Synthesis of 3,5-dichloro-2-fluoropyridine
[0044] Add 2,3,5-trichloropyridine 93.1g (mass fraction 98%, 0.50mol), 300mL sulfolane, powdered potassium fluoride 32.2g (mass fraction 99%, 0.55mol), heated to a temperature of 155-160°C and kept for 8h, sampling analysis, at this time, the content of 2,3,5-trichloropyridine is less than 1.0%, stop the reaction, cool to room temperature, filter, use sulfolane for solid Wash three times (10 mL each), and combine the filtrate and washings.
[0045] (2) Synthesis of 3,5-dichloro-2-cyanopyridine
[0046]Add 1.0g catalyst tetrabutylammonium bromide, 35.5g powdery solid potassium cyanide (massfraction 99%, 0.55mol) in the 3,5-dichloro-2-fluoropyridine solution of step (1) gained, heat To 120-125 ℃ for cyanation reaction. During the reaction, take samples to detect the content changes of intermediates and products, keep warm until the content of i...
Embodiment 2
[0048] The synthetic method of the present embodiment comprises the steps:
[0049] (1) Synthesis of 3,5-dichloro-2-fluoropyridine
[0050] Add 2,3,5-trichloropyridine 93.1g (mass fraction 98%, 0.50mol), 300mL dimethyl sulfoxide, powdered sodium fluoride 23.3 g (mass fraction 99%, 0.55mol), heated to reflux state and maintained for 8h, sampling analysis, at this time, the content of 2,3,5-trichloropyridine was less than 1.0%, stopped the reaction, cooled to room temperature, filtered, and the solid was washed with dichloropyridine Methyl sulfoxide was washed three times (10 mL each), and the filtrate and washings were combined.
[0051] (2) Synthesis of 3,5-dichloro-2-cyanopyridine
[0052] Add 1.0g catalyst benzyltriethylammonium chloride, 27.2g powdered solid sodium cyanide (mass fraction 99%, 0.55mol) to the 3,5-dichloro-2-fluoropyridine solution obtained in step (1) , heated to 125-130 ° C for cyanation reaction. During the reaction, take a sample to detect the change ...
Embodiment 3
[0054] The synthetic method of the present embodiment comprises the steps:
[0055] (1) Synthesis of 3,5-dichloro-2-fluoropyridine
[0056] Add 2,3,5-trichloropyridine 93.1g (mass fraction 98%, 0.50mol), 300mLN,N-dimethylformamide, powdered fluorine Potassium chloride 32.2g (mass fraction 99%, 0.55mol), heated to reflux and kept for 8h, sampling analysis, at this time, the content of 2,3,5-trichloropyridine is less than 1.0%, stop the reaction, cool to room temperature, filter, solid Wash with N,N-dimethylformamide three times (10 mL each time), and combine the filtrate and washings.
[0057] (2) Synthesis of 3,5-dichloro-2-cyanopyridine
[0058] Add 1.0g catalyst tetrabutylammonium bromide, 27.2g powdery solid sodium cyanide (mass fraction 99%, 0.55mol) in the 3,5-dichloro-2-fluoropyridine solution of step (1) gained, heat To reflux for cyanation reaction. During the reaction process, take samples to detect the content changes of intermediates and products, keep warm unti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com