Dibromofluorescein derivative as well as synthetic method and application thereof
A technology of dibromofluorescein and its synthesis method, which is applied in the application field of dibromofluorescein derivatives and its synthesis, and detection of Al3+, achieving the effects of high sensitivity, high specificity and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
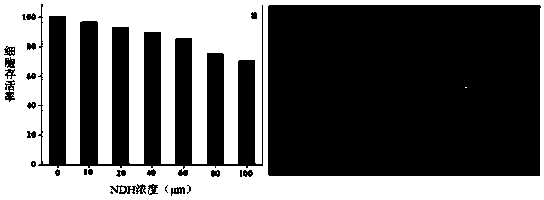
[0029] Synthesis and characterization of embodiment 1 fluorescent probe NDH
[0030] (1) Weigh 3.4g (9.1mmol) of dibromofluorescein and 1mL (20mmol) of hydrazine hydrate solution and dissolve it in 10mL of methanol, react at 65°C for 4 hours, cool to room temperature after the reaction is complete, then rotary evaporate and vacuum Drying and recrystallization from methanol gave a pink solid.
[0031] (2) Weigh 0.019g (0.05mmol) of the product obtained above and 0.0075g (0.05mmol) of 2-hydroxy-5-nitrobenzaldehyde into 10mL of absolute ethanol, and ultrasonically dissolve it for 5min, then stir for 65 Reflux at °C for 4 hours, cool to room temperature after the reaction is complete, then rotary evaporate and vacuum-dry, and then recrystallize with methanol to obtain off-white solid powder. 1H NMR(600MHz,DMSO)δ12.23(s,1H),9.97(s,2H),9.11(s,1H),8.72(d,J=2.8Hz,1H),8.28(d,J=9.1Hz ,1H),7.99(d,J=32.5Hz,1H),7.18(d,J=9.1Hz,1H),7.00(d,J=9.3Hz,1H),6.68(d,J=8.8Hz,1H ), 6.43 (q, J=8.6Hz,...
Embodiment 2
[0032] Embodiment 2 fluorescent probe NDH is used for Al 3+ UV absorbance determination
[0033] Take 20 μL of the storage solution of the fluorescent probe NDH in a clean colorimetric tube, dilute it to 2 mL with THF:phosphate buffer solution (9:1), and scan the absorption spectrum on a UV-visible spectrophotometer. It can be observed that in the visible region There is absorption at 443nm, adding Al 3+ After that, the absorption peak at 443nm disappears see figure 1 , and the color of the solution changed from yellow to colorless, and the presence of aluminum ions could be judged with the naked eye.
Embodiment 3
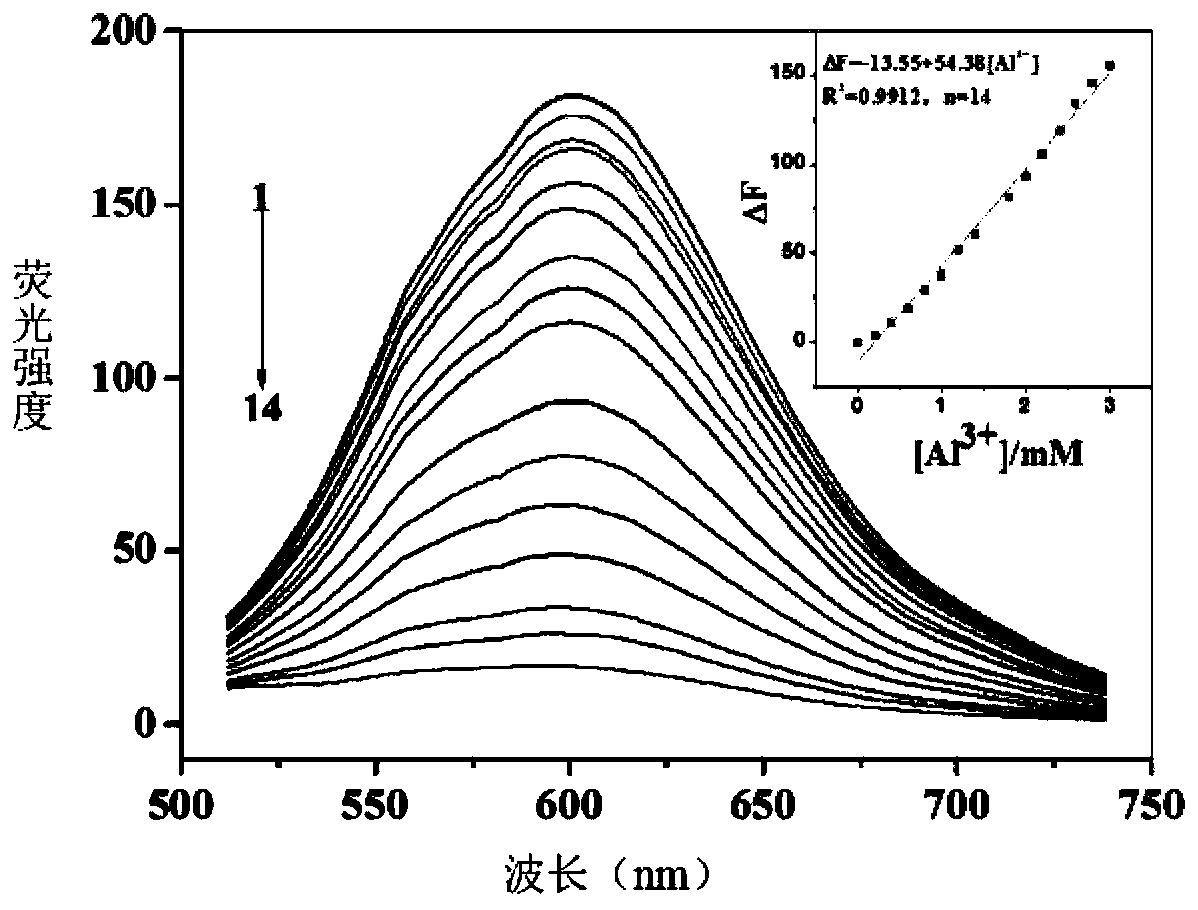
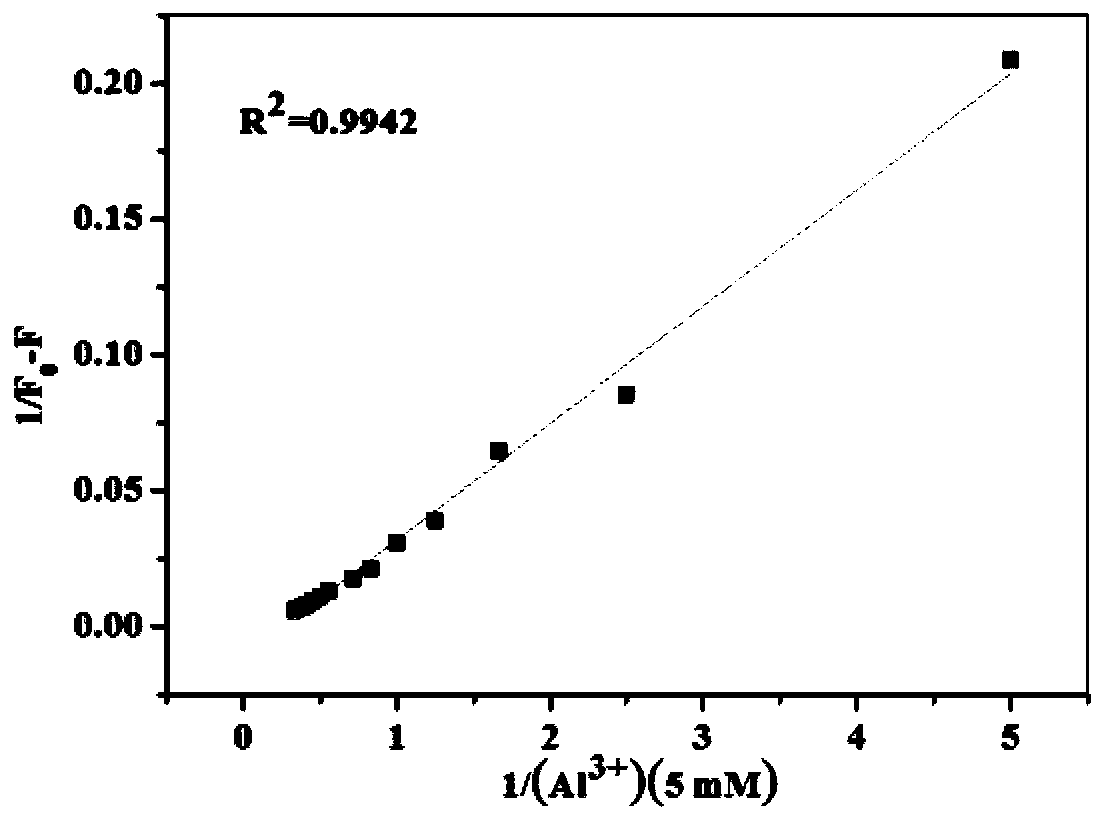
[0034] Embodiment 3 fluorescent probe NDH is used for Al 3+ Fluorescence spectrometry
[0035] Take 20 μL of the storage solution of the fluorescent probe NDH in a clean colorimetric tube, dilute it to 2 mL with THF:phosphate buffer solution (9:1), and detect it on a fluorescence photometer. When the excitation wavelength is 445 nm, dibromofluorescein The derivative has an emission peak at 600nm, when adding Al 3+ After that, the intensity of the fluorescence emission peak at 600nm decreased gradually. figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com