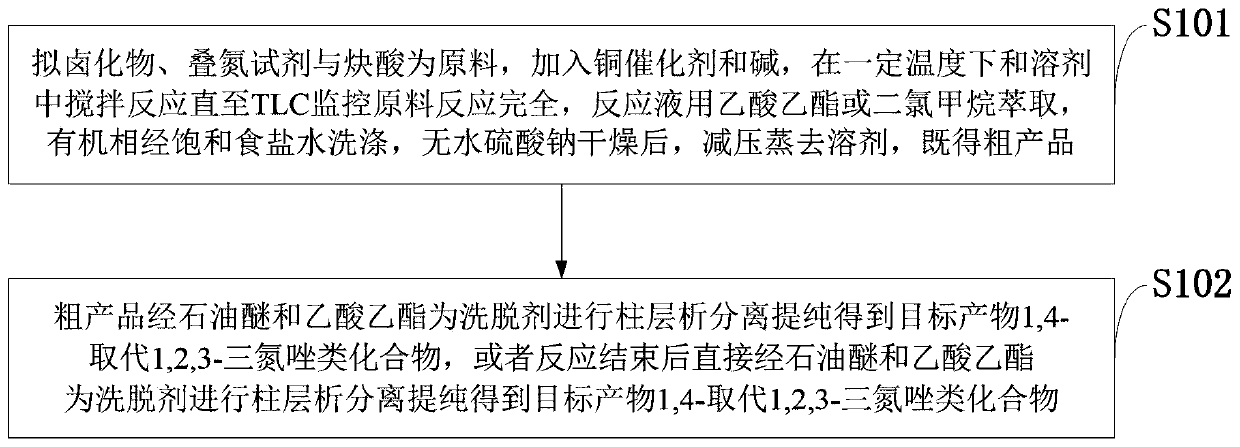
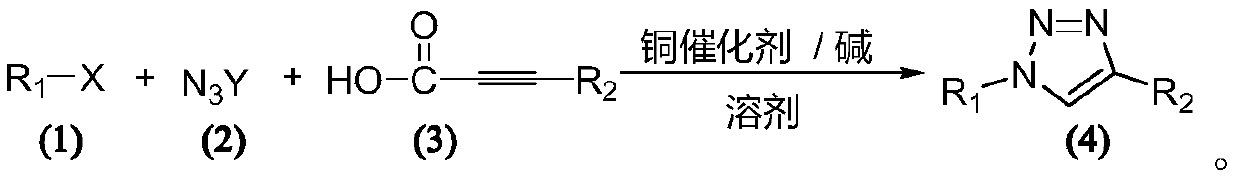
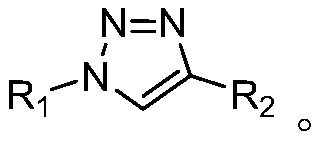
Method for synthesizing 1,4-substituted 1,2,3-triazole by alkynoic acid multi-components
A multi-component, triazole technology, applied in the direction of chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve the problems of limited application range, long reaction time, large limitations, etc., and achieve easy storage Easy to transport, easy to handle, and easy to synthesize effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1: Add 1.0mmol of phenylpropiolic acid, 1.5mol of sodium azide, 1.2mmol of m-trifluorobenzyl bromide, 0.2mmol of potassium carbonate, and 0.1mmol of cuprous iodide to polyethylene glycol 4003.0mL, 60 ℃ stirring reaction for 30min, after the reaction, the product was subjected to column chromatography V 石油醚 :V 乙酸乙酯 = 3:1 treatment gives 1-(3-trifluoromethylbenzyl)-4-phenyl-1,2,3-triazole. The product is a white solid, yield: 98%.
[0074]
Embodiment 2
[0075] Example 2: Add 1.0mmol of phenylpropiolic acid, 1.5mol of sodium azide, 1.2mmol of m-trifluorobenzyl bromide, and 0.1mmol of cuprous iodide into polyethylene glycol 4003.0mL, and stir at 60°C for 2.0h , after the reaction, the product is subjected to column chromatography V 石油醚 :V 乙酸乙酯 = 3:1 treatment gives 1-(3-trifluoromethylbenzyl)-4-phenyl-1,2,3-triazole. The product is a white solid, yield: 89%.
[0076]
Embodiment 3
[0077] Example 3: 1.0mmol of phenylpropiolic acid, 1.5mol of sodium azide, 1.2mmol of m-trifluorobenzyl bromide, and 0.1mmol of cuprous iodide were added to polyethylene glycol 4003.0mL, stirred at room temperature for 25h, and the reaction After the end, the product was subjected to column chromatography V 石油醚 :V 乙酸乙酯 = 3:1 treatment gives 1-(3-trifluoromethylbenzyl)-4-phenyl-1,2,3-triazole. The product is a white solid, yield: 85%.
[0078]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com