Poly-valerolactone type amphiphilic polymer based on tetraphenyl ethylene as well as preparation method and application of poly-valerolactone type amphiphilic polymer
A technology of polyvalerolactone and tetraphenylethylene, which is applied in the field of polymer design and preparation, and can solve the problems of great difference in transfection efficiency and single function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] A polyvalerolactone type amphiphilic polymer based on tetraphenylethylene, the structural formula (I) of the compound is as follows:
[0051]
[0052] In formula (I), R 1 is a sulfur-containing substituent, R 2 is a substituted macrocyclic polyamine [12]aneN 3 group, m, n are positive integers.
[0053] Among them, when R 1 for R2 for Denoted as polymer 1;
[0054] When R 1 for a=6, R 2 for Denoted as polymer 2;
[0055] When R 1 for a=10,R 2 for Denoted as polymer 3;
[0056] Concrete synthetic route is as follows:
[0057]
[0058]
[0059] The synthetic procedure of compound 1-1: have 3.0g (16.5mmol) [12]aneN to dissolve 3 Add 10.0 mL of 1,3-dibromopropane (98.5 mmol) to 30.0 mL of the precursor solution in acetonitrile, stir and reflux overnight; stop the reaction when a large amount of white solid appears in the bottle. Acetonitrile was removed by rotary evaporation under reduced pressure to obtain a yellow solid-liquid mixture. Ad...
Embodiment 2
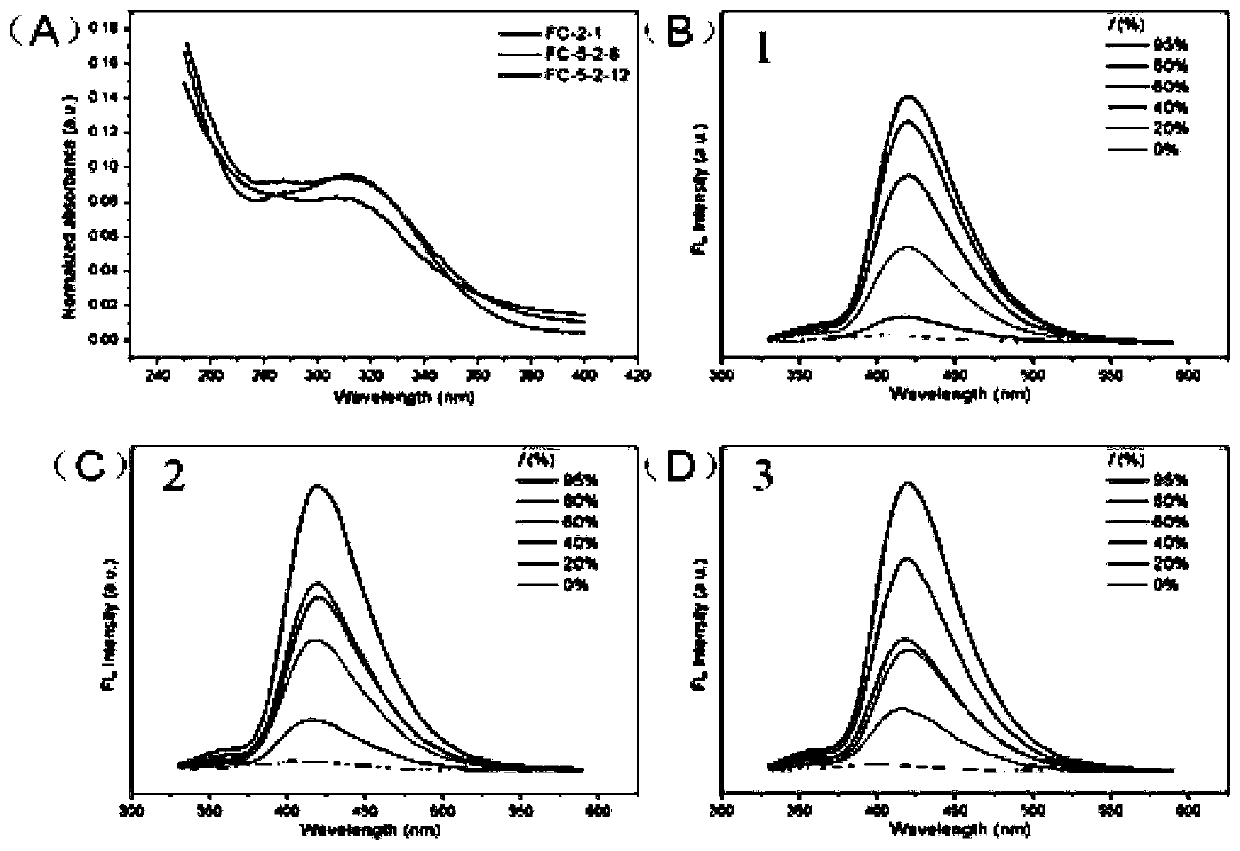
[0066] Polymers 1, 2 and 3 were configured into aqueous solutions of different concentrations, and the fluorescence intensity of the aqueous solutions of polymers 1 to 3 was measured; the maximum value of the fluorescence intensity varied with the concentration (10 -6 ~10 -1 mg / mL) change trend graph, get figure 1 A~ figure 1 C, at figure 1 In A-1C, the X-axis is the solution concentration, and the Y-axis is the fluorescence intensity.
[0067] figure 1 A~1C are the determination diagrams of the critical micelle concentration of polymers 1~3 respectively, from figure 1 From A to 1C, it can be seen that the critical micelle concentrations of compounds 1 to 3 are very low, and the critical micelle concentrations of compounds 1 to 3 are 9.6 μg / mL, 0.5 μg / mL, and 1.1 μg / mL, respectively
Embodiment 3
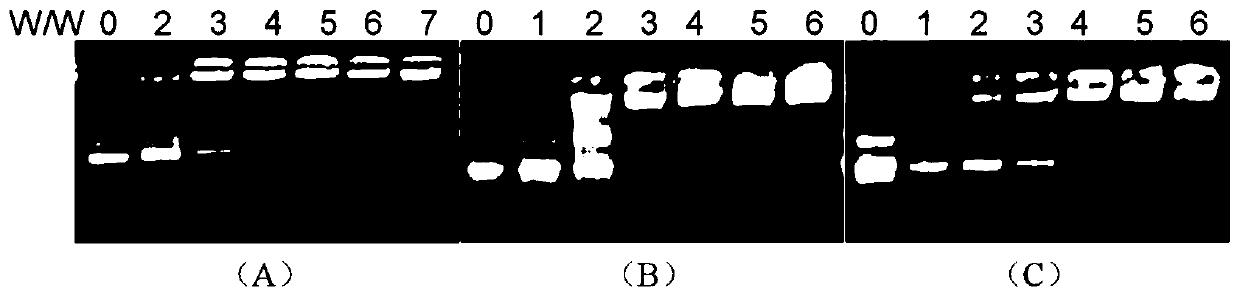
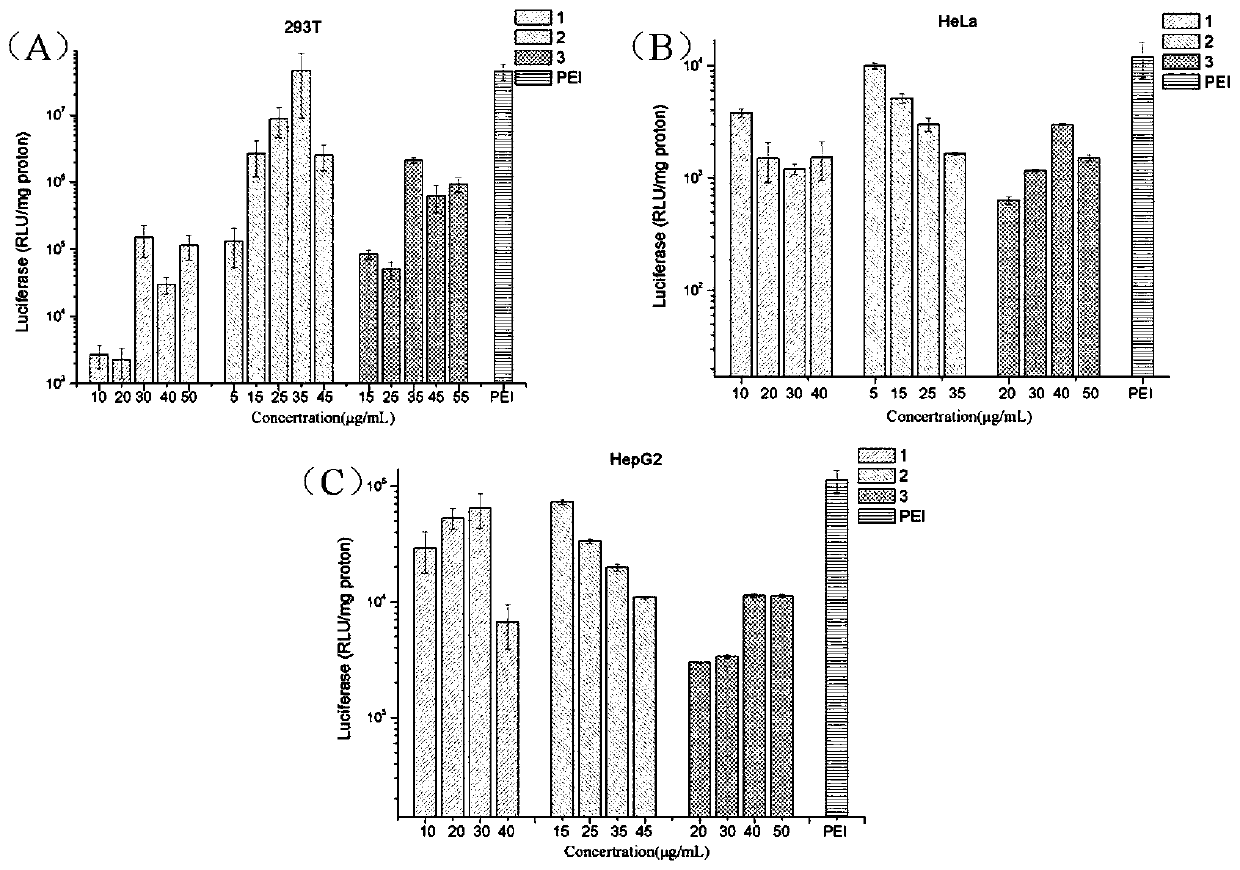
[0069] Prepare solutions of different concentrations of 1 / DOPE, 2 / DOPE and 3 / DOPE (1:2, molar ratio), form complexes with pGL-3 plasmid DNA, incubate at 37°C for 0.5 hours, and then Add it into different gel wells, and carry out DNA agarose gel retardation experiment to obtain the aggregation of DNA by different concentrations of polymers.
[0070] figure 2 A~2C are respectively the agarose gel retardation experiment results of the pGL-3DNA of the polymers 1 / DOPE, 2 / DOPE and 3 / DOPE of the present invention; figure 2 It can be drawn that the tetraphenylethylene-based polyvalerolactone amphiphilic polymer of the present invention can effectively condense DNA to form nanoparticles, and the minimum blocking concentrations of polymers 1 to 3 are respectively: 40 μg / mL, 30 μg / mL and 40 μg / mL.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com