Preparation method of amorphous iron oxyhydroxide and recovery method after absorbing organic matter
An iron oxyhydroxide and amorphous technology, which is applied in the fields of iron oxide, chemical instruments and methods, iron oxide/iron hydroxide, etc., can solve the problems of low production efficiency and difficult recycling of iron oxyhydroxide, and achieve the improvement of production efficiency, Improve preparation efficiency and good catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
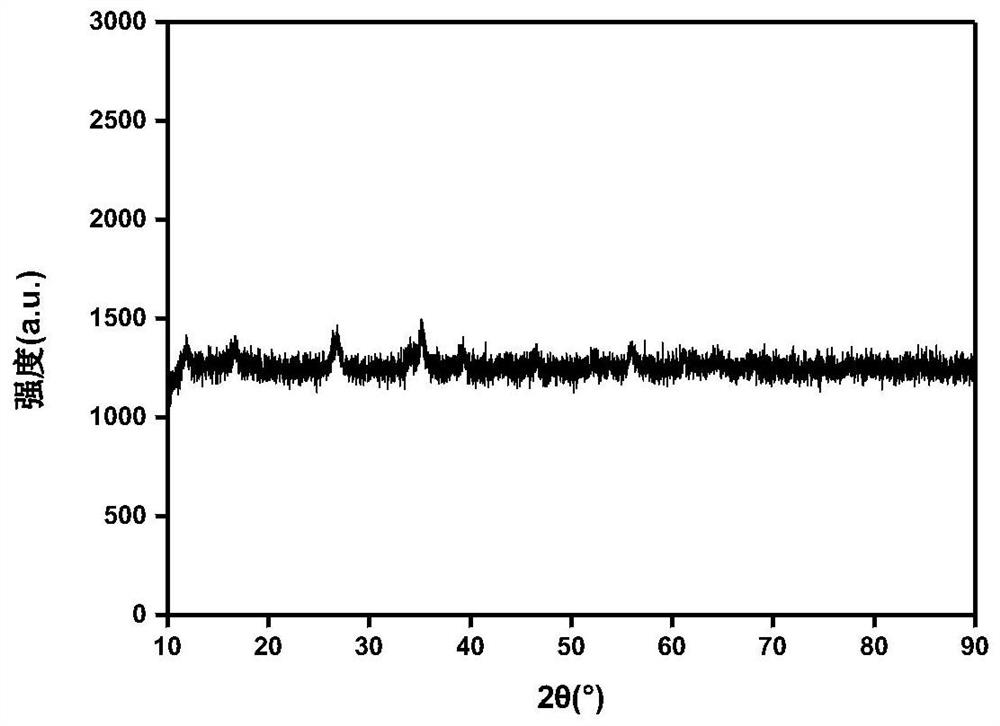
[0021] Make kerosene, Span 80, Tween 80 and distilled water into a microemulsion phase according to the mass ratio: 20:10:10:1, stir until transparent; add ferric chloride powder, ferric chloride powder and distilled water The mass ratio is 1-4:1, and it is fully stirred until the powder is completely dissolved, adding sodium hydroxide particles, the molar ratio of sodium hydroxide to ferric chloride is 1:1, heating to 80 ° C for 30 minutes and then stopping heating, The precipitate was obtained by centrifugation, washed twice with absolute ethanol, washed twice with distilled water, and dried to obtain amorphous iron oxyhydroxide. Its XRD pattern is attached figure 1 shown.
[0022] Add 0.1 g of amorphous ferric oxyhydroxide to 100 ml of methyl orange solution with a concentration of 50 mg / l, stir and adsorb for 10 minutes under the condition of avoiding light, take a sample, and centrifuge for 10 minutes at a speed of 10,000 rpm in a centrifuge , remove the amorphous iron ...
Embodiment 2
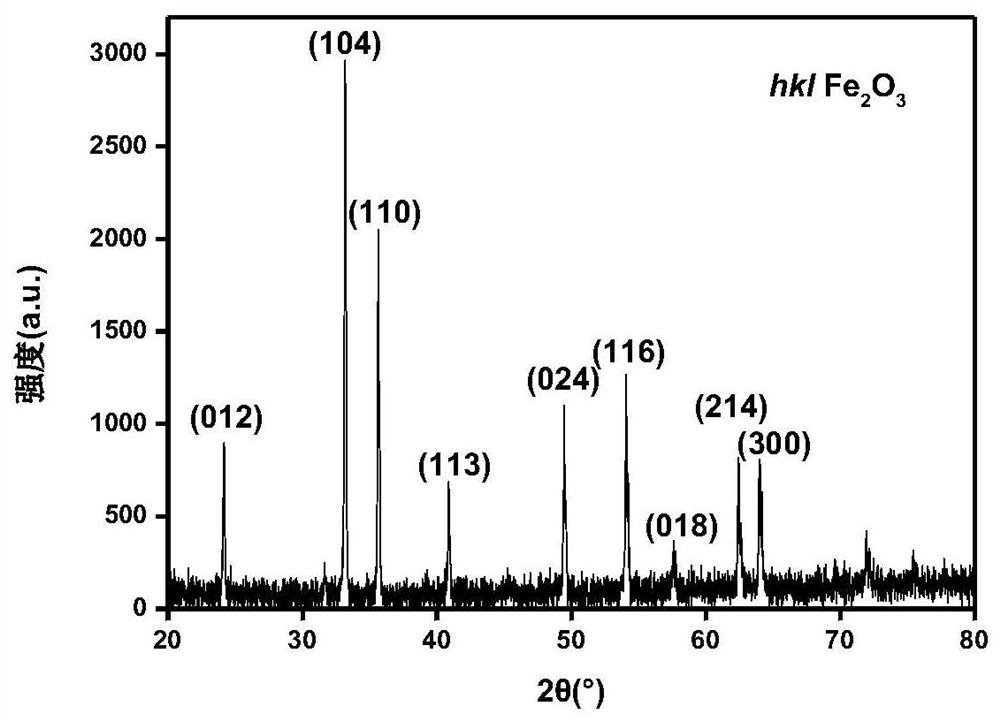
[0024] The amorphous iron oxyhydroxide adsorbed methyl orange in Example 1 was calcined at 700°C for 2 hours to obtain nano-α iron oxyhydroxide, and its XRD pattern is as attached image 3 shown. Take 100 milliliters of methyl orange solution with a concentration of 20 mg / l, add 0.01 g of nano-α ferric oxyhydroxide, stir for 1 hour in the dark, add 5 milliliters of 30% hydrogen peroxide, and irradiate it under 300 nm ultraviolet light for degradation. The degradation rate is shown in the attached Figure 4 shown.
Embodiment 3
[0026] Take 100 milliliters of methyl orange solution with a concentration of 20 mg / l, add 0.01 g of nano-α ferric oxyhydroxide prepared in Example 2, and stir in the dark for 1 hour, add 5 milliliters of 30% hydrogen peroxide, and irradiate it under a 300W xenon lamp. Degradation, degradation rate as attached Figure 5 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com