Preparation and application of coumarin type fluorescent probe for detecting Hg<2+>
A fluorescent probe and coumarin-based technology, applied in the direction of fluorescence/phosphorescence, luminescent materials, measuring devices, etc., can solve the problems of complex operation process equipment, not suitable for in-situ analysis, etc., and achieve wide application prospects and good selectivity and the effect of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Preparation of 7-(diethylamino)-3-(1,3-dithian-2-yl)-2H-pyran-2-one
[0018]
[0019] Add 5mmol, 965mg of 4-diethylaminosalicylaldehyde into 20.0mL of ethanol to make it fully dissolved, then add 10mmol, 1.56mL of diethyl malonate and 5mmol, 494μL of piperidine, the amount of substance The ratio of 4-diethylaminosalicylaldehyde: diethyl malonate: piperidine=1:2:1, the reaction mixture was refluxed for 10h, monitored by TLC, the solvent was removed under reduced pressure, and then 10.0mL of concentrated hydrochloric acid and 10.0mL of glacial acetic acid was hydrolyzed, and stirred at 100°C for 6h, the solution was cooled to room temperature and poured into 50mL of ice water, 50% NaOH solution was added to adjust the pH to 7.0-8.0, then dichloromethane was added, and the organic layer was washed with water three times, Dry with anhydrous sodium sulfite, concentrate under reduced pressure, the crude product is purified by silica gel column chromatography, th...
Embodiment 2
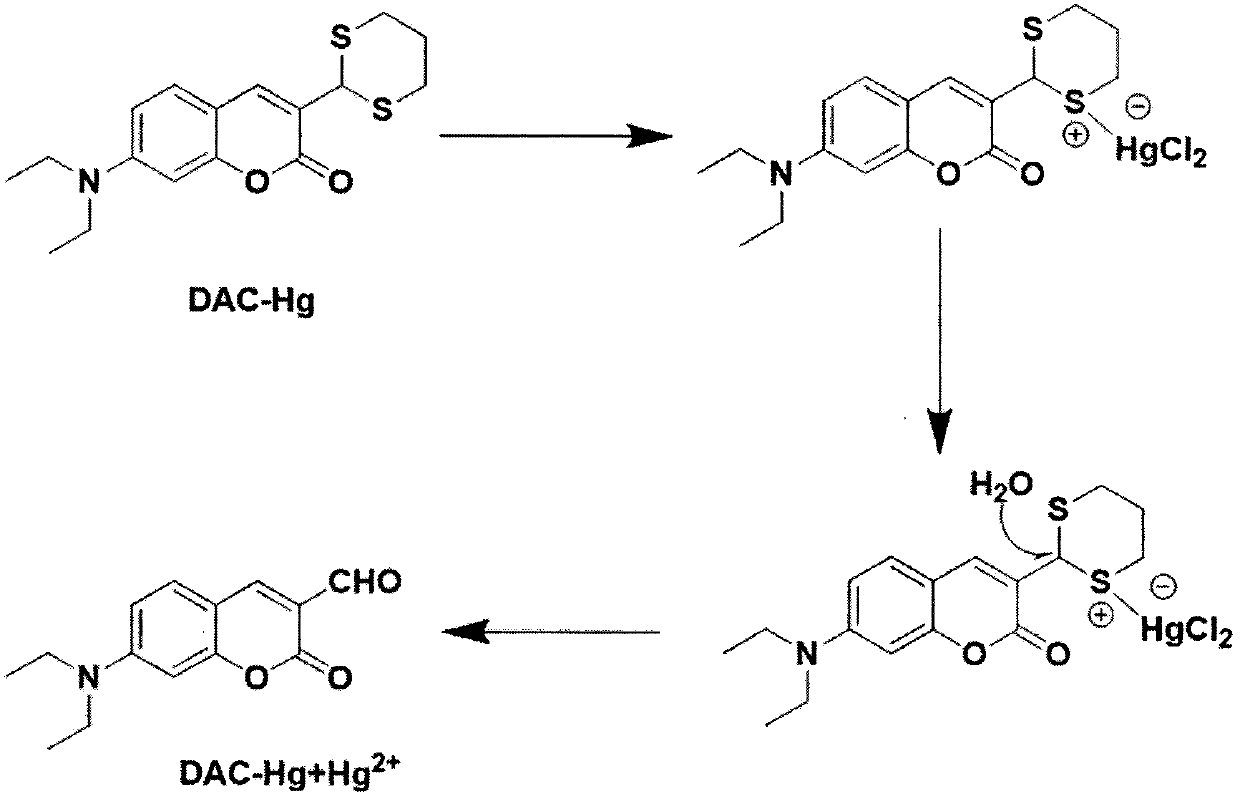
[0022] figure 1 : Target compound detection Hg 2+ Mechanism research
Embodiment 3
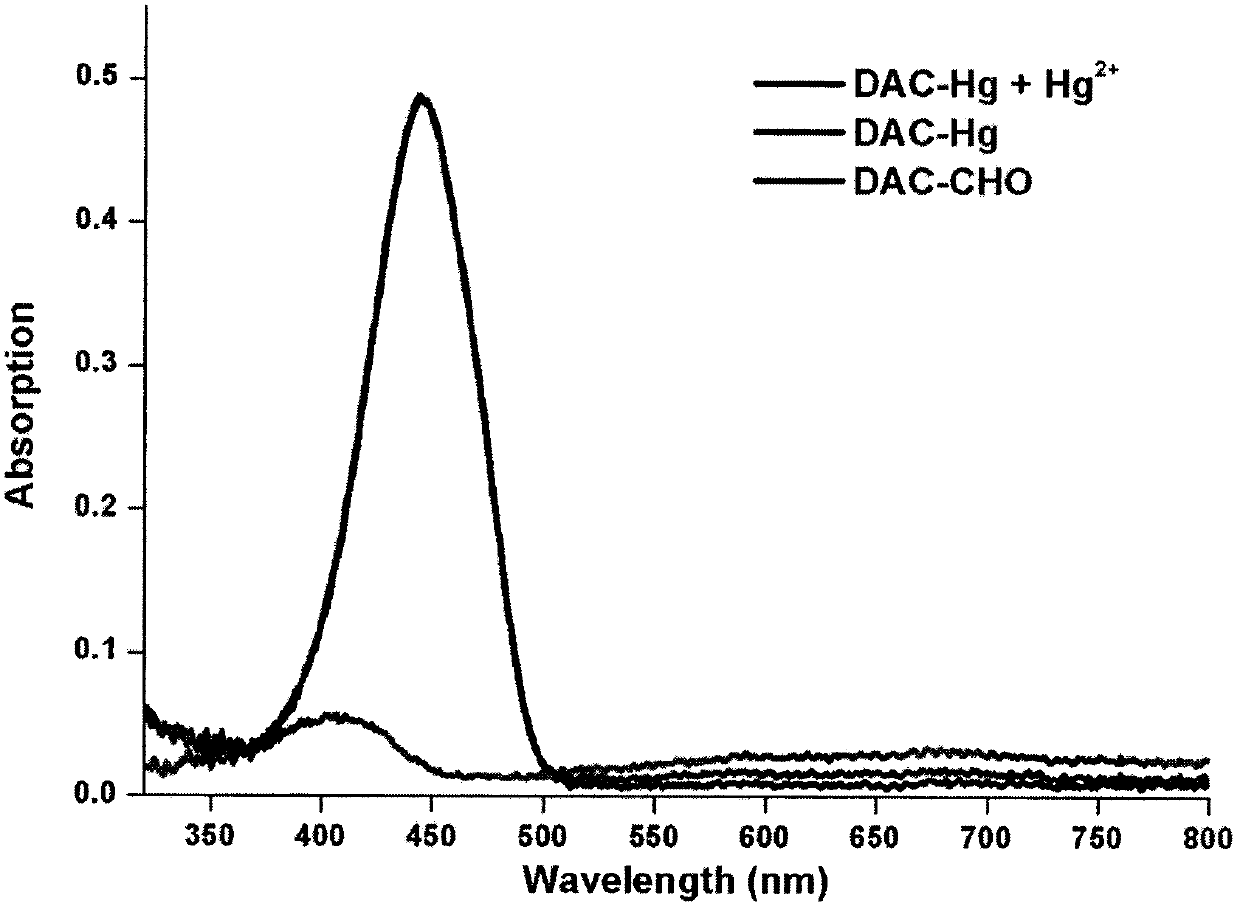
[0024] figure 2 : In PBS buffer, the fluorescent molecular probe and Hg 2+ UV / Vis absorption spectrum after reaction
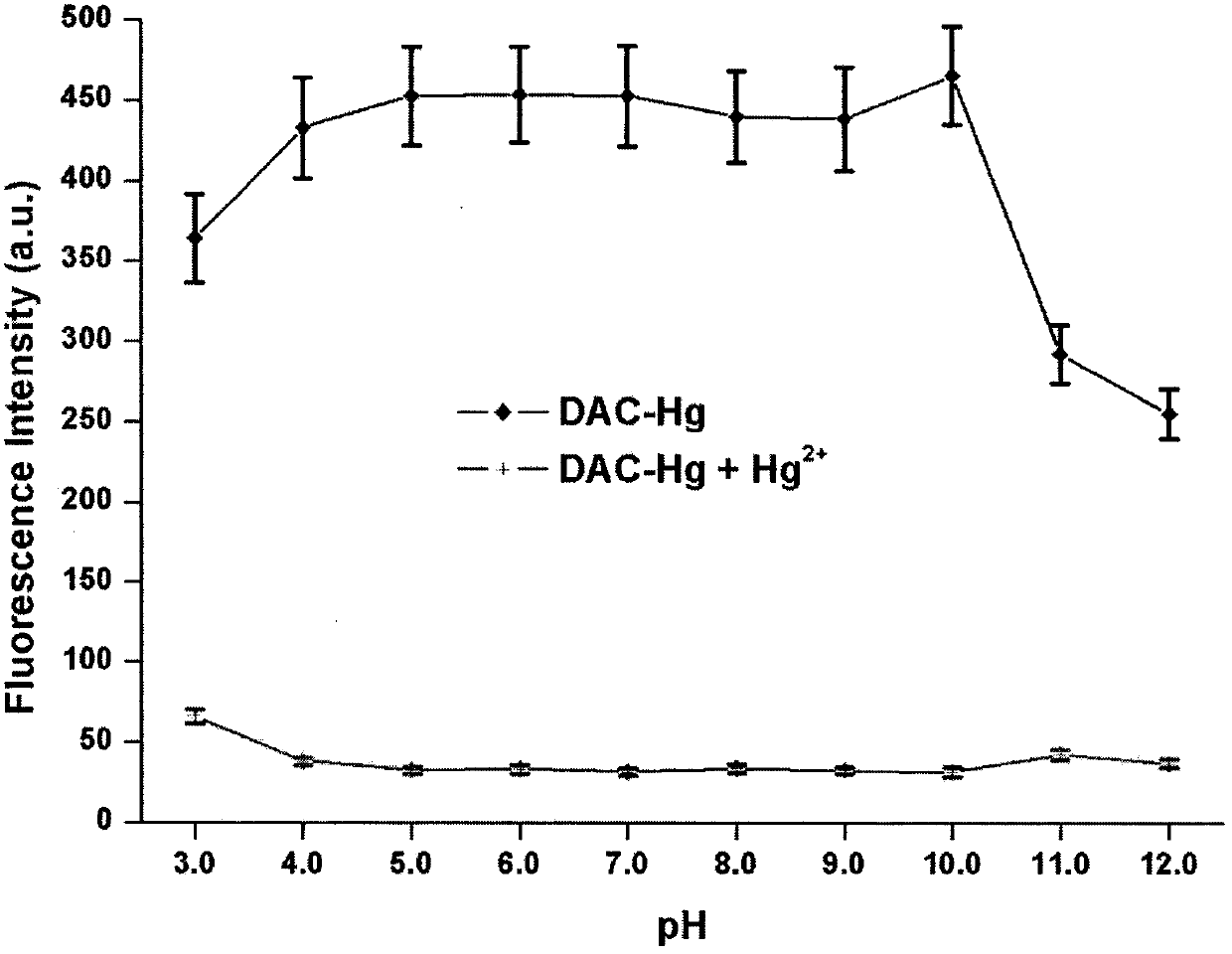
[0025] The emission spectrum of free DAC-Hg (10 μM) in PBS buffer (10 mM, 10% DMSO, pH 7.4) was collected 25 times. The excitation wavelength was 400nm, the voltage of the photomultiplier tube was set at 500V, and the slit width was set at 5.0nm, and the detection was performed on a Shimadzu UV-2550 instrument.
[0026] The results showed that the free probe DAC-Hg showed a peak at 403nm, while DAC-CHO and DACHg+Hg 2+ Both show a peak at 444nm. The fluorescence quantum yield of DAC-Hg determined subsequently was 0.93, and the detected product DAC-CHO was 0.27.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com