Efficient nano-catalyst for hydrogen production by formic acid hydrolysis and preparation method thereof
A technology for hydrogen production and catalysts by hydrolysis, which is applied in the direction of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., which can solve the problems of increased catalytic efficiency, achieve improved dispersion, good hydrogen selectivity, and reduce particles effect of size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 1. Preparation of high-efficiency catalyst:
[0055] Disperse 30mg of GO in 20mL of ultrapure water, sonicate for 15min, add 0.4ml of APTS, stir evenly; take 0.035mmol of HAuCl respectively 4 solution, 0.05mmol of Na 2 PdCl 4 solution and 0.015 mmol of IrCl 3 ·xH 2 O solution was dissolved in APTS+GO aqueous solution, stirred for 3min; at 25°C, 30mg of NaBH was added 4 , the mixed solution was magnetically stirred evenly until no bubbles were generated, and it was completely reduced; after centrifugation and washing with water, Au 0.35 PD 0.5 Ir 0.15 / NH 2 -N-rGO catalyst.
[0056] 2. Sample testing:
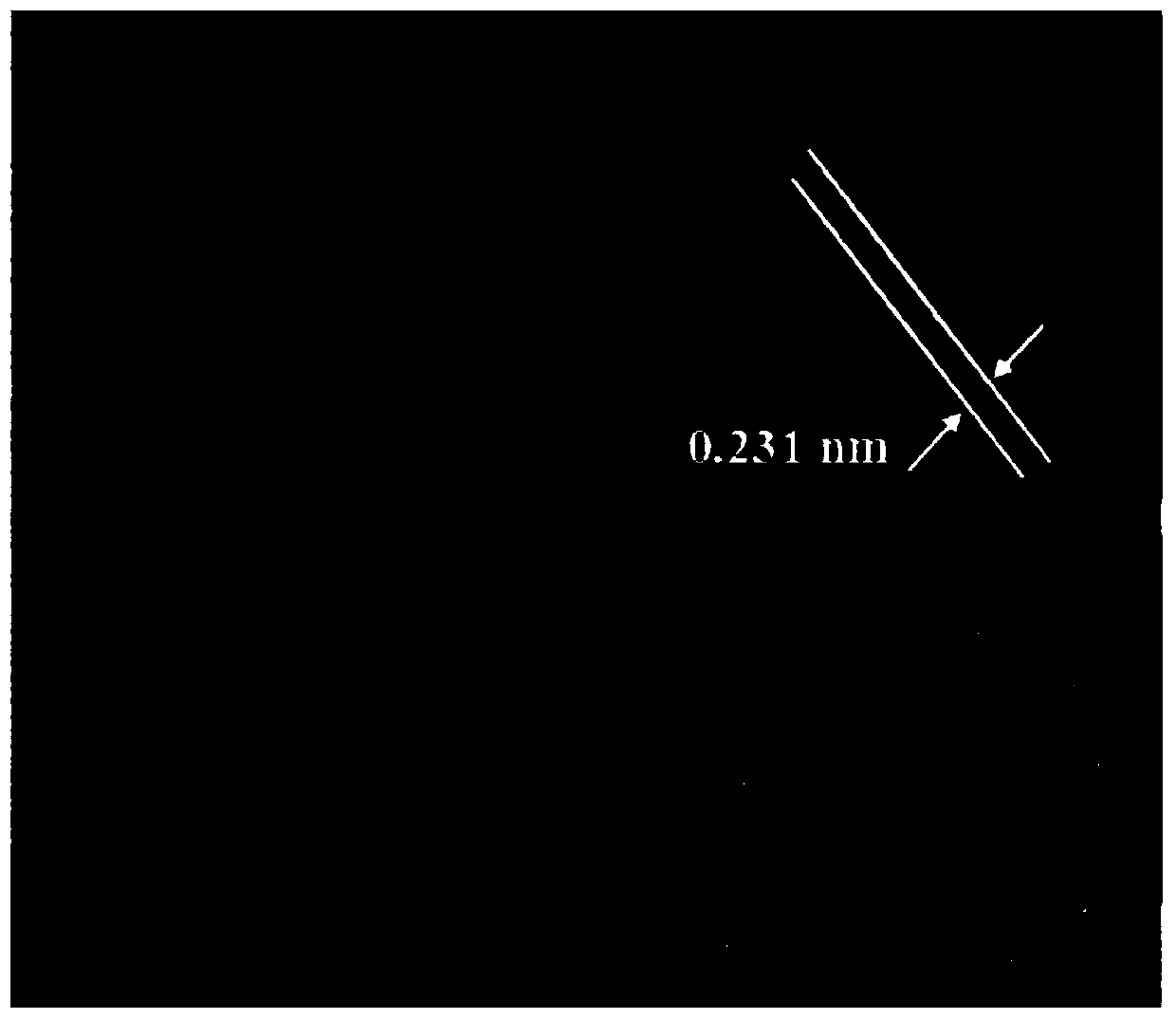
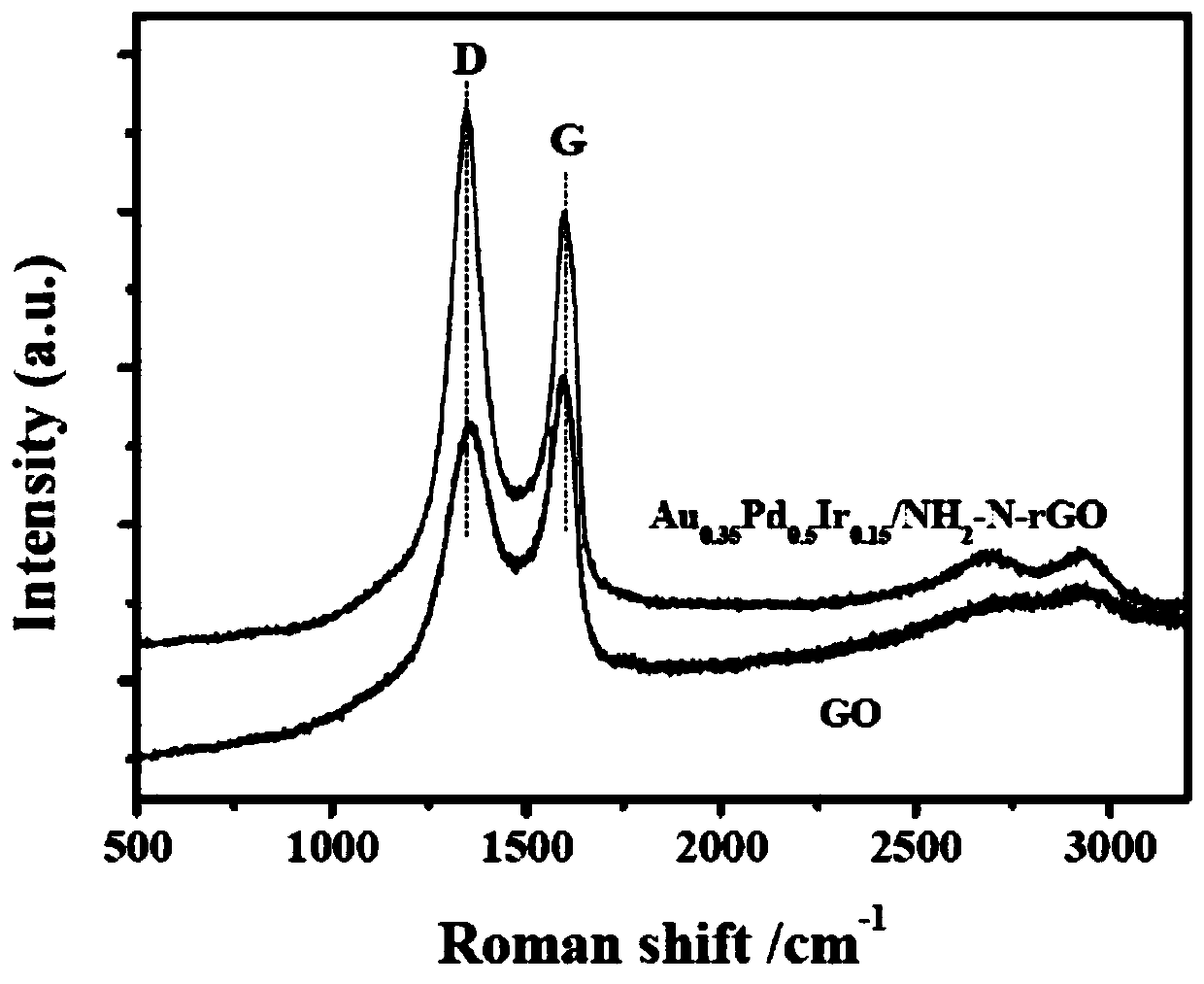
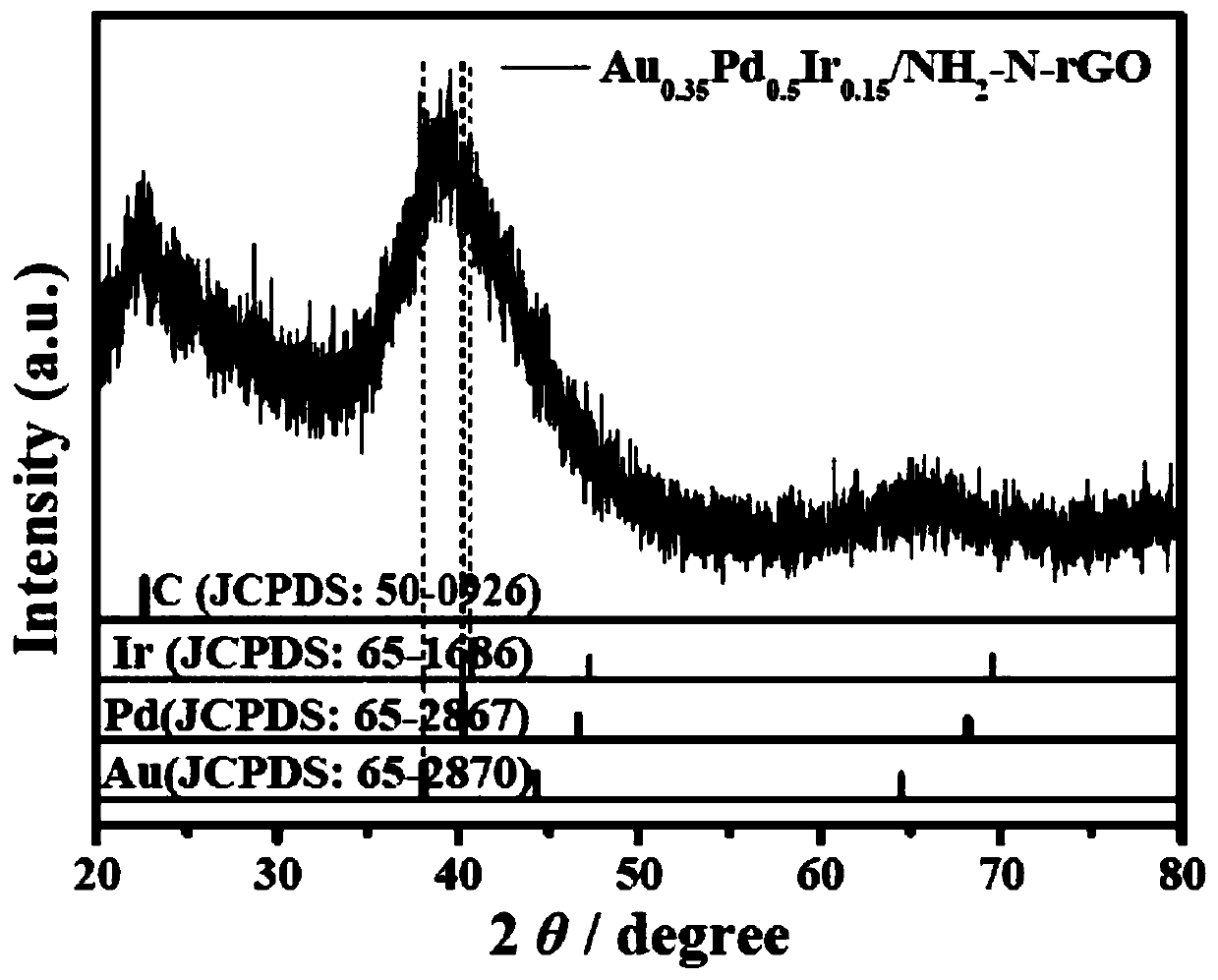
[0057] (1) The prepared Au 0.35 PD 0.5 Ir 0.15 / NH 2 - N-rGO catalyst diluted, dropped on carbon support film, dried; ref. figure 1 , the results of transmission electron microscopy (TEM) and particle size analysis showed that Au 0.35 PD 0.5 Ir 0.15 / NH 2 -N-rGO samples have ultra-fine particle size (2.58nm) and uniform dispersion. High-resolution transm...
Embodiment 2
[0063] 1. Preparation of high-efficiency catalyst:
[0064] Disperse 30mg of GO in 20mL of ultrapure water, sonicate for 15min, add 0.4ml of APTS, stir evenly; take 0.03mmol of HAuCl respectively 4 solution, 0.05mmol of Na 2 PdCl 4 solution and 0.02mmol IrCl 3 ·xH 2 O solution was dissolved in APTS+GO aqueous solution, stirred for 3min; at 25°C, 30mg of NaBH was added 4 , the mixed solution was magnetically stirred evenly until no bubbles were generated, and it was completely reduced; after centrifugation and washing with water, Au 0.3 PD 0.5 Ir 0.2 / NH 2 -N-rGO catalyst.
[0065] 2. Sample testing:
[0066] (1) The prepared Au 0.3 PD 0.5 Ir 0.2 / NH 2 - N-rGO catalyst diluted, dropped on carbon support film, dried; ref. Figure 5 , the results of transmission electron microscopy (TEM) showed that Au 0.3 PD 0.5 Ir 0.2 / NH 2 -N-rGO samples have ultrafine particle size and uniform dispersion.
[0067] (2) the prepared Au 0.3 PD 0.5 Ir 0.2 / NH 2 -N-rGO catal...
Embodiment 3
[0072] 1. Preparation of high-efficiency catalyst:
[0073] Disperse 30mg of GO in 20mL of ultrapure water, sonicate for 15min, add 0.4ml of APTS, stir evenly; take 0.0417mol of HAuCl respectively 4 solution, 0.05mol Na 2 PdCl 4 solution and 0.0083mol of IrCl 3 ·xH 2 O solution was dissolved in APTS+GO aqueous solution, stirred for 3min; at 25°C, 30mg of NaBH was added 4 , the mixed solution was magnetically stirred evenly until no bubbles were generated, and it was completely reduced; after centrifugation and washing with water, Au 0.5 PD 0.6 Ir 0.1 / NH 2 -N-rGO catalyst.
[0074] 2. Sample testing:
[0075] (1) The prepared Au 0.5 PD 0.6 Ir 0.1 / NH 2 - N-rGO catalyst diluted, dropped on carbon support film, dried; ref. Figure 9 , the results of transmission electron microscopy (TEM) showed that Au 0.5 PD 0.6 Ir 0.1 / NH 2 -N-rGO samples have ultrafine particle size and uniform dispersion.
[0076] (2) the prepared Au 0.5 PD 0.6 Ir 0.1 / NH 2 -N-rGO cata...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com