Method for synthesizing 1,2-dissubstituted olefin compounds by alkyne alkylation
A compound and disubstitution technology, applied in the field of synthesis of organic compounds, can solve problems such as addition that has not been reported, and achieve high stereoselectivity, high yield, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]
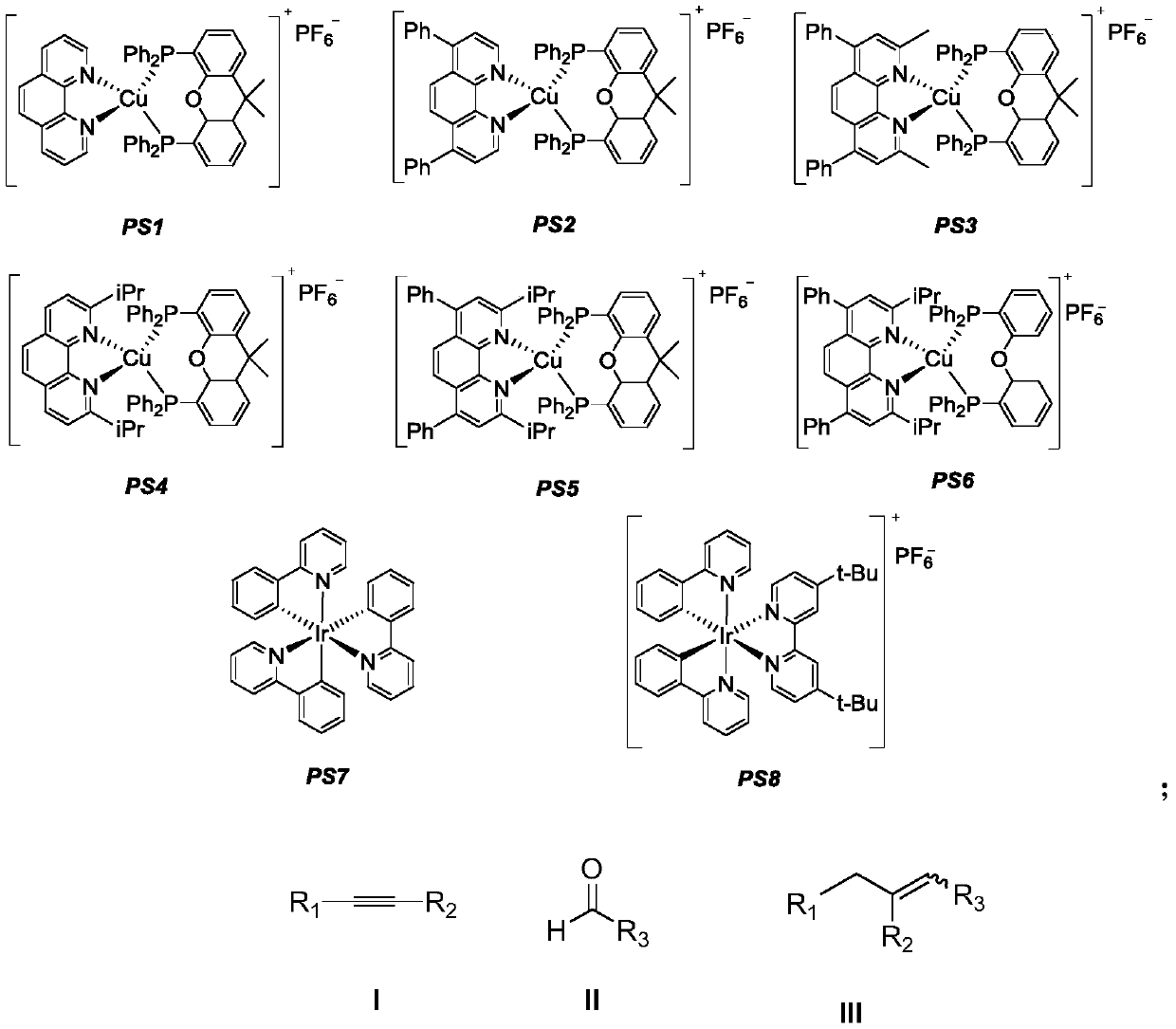
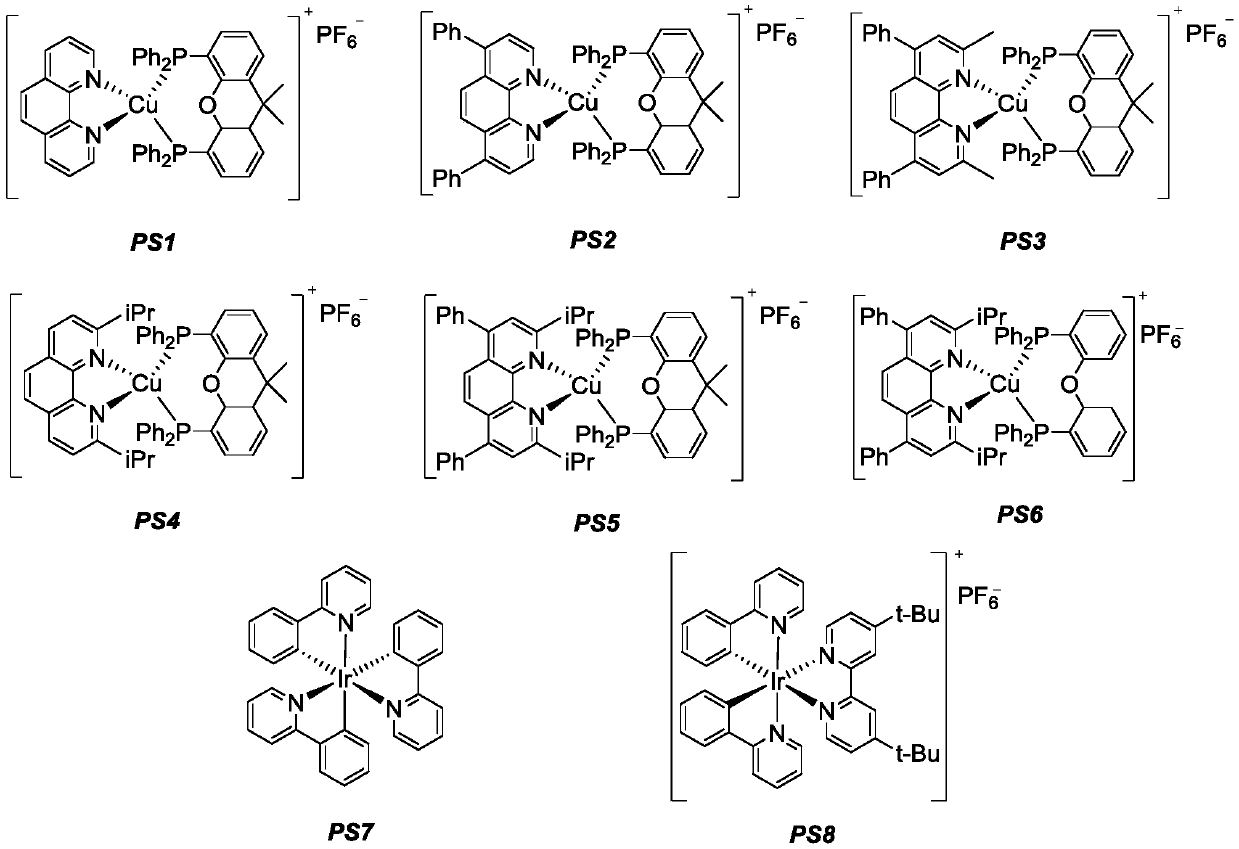
[0039] The photosensitizer PS3 (0.01mmol, 11.5mg), 4-phenylphenylacetylene (0.2mmol, 35.6mg), Hans ester (0.24mmol, 60.8mg) was added to a dry Schlenk reaction tube, and the nitrogen gas was replaced three times. Under nitrogen protection, butyraldehyde (1.0 mmol, 72.1 mg) and diethylamine (1.0 mmol, 73.1 mg) in acetonitrile (4 mL) were added, and the reaction tube was stirred for 12 hours under 15W blue LED irradiation. After the reaction was completed, 100-200 mesh column chromatography silica gel was added to the obtained reaction solution and the solvent was distilled off under reduced pressure, and the obtained crude product was subjected to silica gel column chromatography separation, and eluted with petroleum ether as an eluent, followed by TLC. In the elution process, collect the eluent containing the target product, combine the eluents and evaporate the solvent to obtain the pure product. The material was a colorless liquid, 15% yield, E / Z=10 / 1 of the prod...
Embodiment 2
[0045]
[0046] The photosensitizer PS4 (0.01mmol, 10.5mg), phenylacetylene (0.2mmol, 20.4mg), Hans ester (0.24mmol, 60.8mg) was added to the dry Schlenk reaction tube, filled with nitrogen three times, under the protection of nitrogen Butyraldehyde (1.0 mmol, 72.1 mg) and diethylamine (1.0 mmol, 73.1 mg) in acetonitrile (4 mL) were added, and the reaction tube was stirred for 12 hours under 15W blue LED irradiation. After the reaction was completed, 100-200 mesh column chromatography silica gel was added to the obtained reaction solution and the solvent was distilled off under reduced pressure, and the obtained crude product was subjected to silica gel column chromatography separation, and eluted with petroleum ether as an eluent, followed by TLC. In the elution process, collect the eluent containing the target product, combine the eluents and evaporate the solvent to obtain the pure product. The material was a colorless liquid, yield 14%, E / Z=10 / 1 of the product.
[0047...
Embodiment 3
[0049]
[0050] The photosensitizer PS5 (0.01mmol, 12.0mg), 4-bromophenylacetylene (0.2mmol, 36.2mg), Hans ester (0.24mmol, 60.8mg) was added to the dry Schlenk reaction tube, and the nitrogen gas was replaced three times. Under nitrogen protection, butyraldehyde (1.0 mmol, 72.1 mg) and diethylamine (1.0 mmol, 73.1 mg) in acetonitrile (4 mL) were added, and the reaction tube was placed under 15W blue LED irradiation and stirred for 12 hours. After the reaction was completed, 100-200 mesh column chromatography silica gel was added to the obtained reaction solution and the solvent was distilled off under reduced pressure, and the obtained crude product was subjected to silica gel column chromatography separation, and eluted with petroleum ether as an eluent, followed by TLC. In the elution process, collect the eluent containing the target product, combine the eluents and evaporate the solvent to obtain the pure product. The material was a colorless liquid, 27% yield, E / Z=11 / 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com