Nosiheptide soluble powder and preparation method thereof
A nosiheptide, soluble technology, applied in the field of nosiheptide soluble powder and its preparation, can solve the problems of non-soluble nosiheptide difficult to dissolve and absorb, low dose of nosiheptide, low bioavailability, etc., to achieve good absorption The effect of utilization, improvement of bioavailability, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]The carrier is selected from hydroxypropyl-β-cyclodextrin and polyamide-amine, the cosolvent is selected from poloxamer 188 and PVPK30, and the stabilizer is selected from vitamin E.
[0049] The preparation method of nosiheptide soluble powder is:
[0050] (1) Take 34.5 parts of hydroxypropyl-β-cyclodextrin, take 15.0 parts of poloxamer 188, 15.0 parts of PVPK30, weigh 34.5 parts of polyamide-amine, and 1.0 parts of vitamin E, and dissolve them in water to obtain a mixed solution .
[0051] (2) Add 0.5 part of nosiheptide to the above mixed solution, and dissolve;
[0052] (3) spray drying.
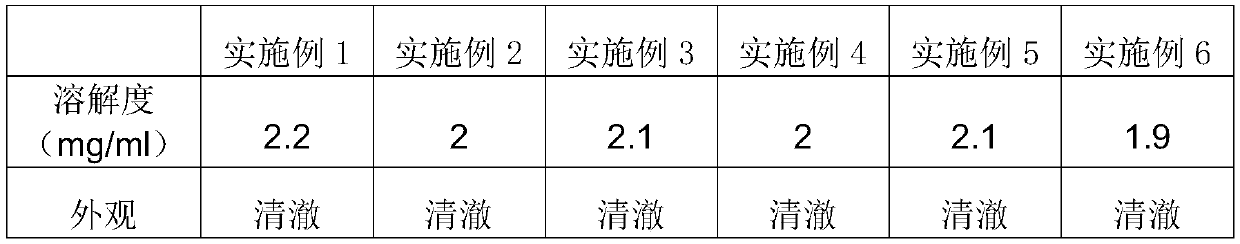
[0053] The Nosiheptide soluble powder obtained in this example has a solubility of Nosiheptide in water of 2.2% at room temperature.
Embodiment 2
[0055] The carrier is selected from hydroxypropyl-β-cyclodextrin, the cosolvent is selected from poloxamer 188 and PVPK30, and the stabilizer is selected from vitamin E and L-cystine.
[0056] The preparation method of nosiheptide soluble powder is:
[0057] (1) Take 69 parts of hydroxypropyl-β-cyclodextrin, take 10.0 parts of poloxamer 188, 20.0 parts of PVPK30, and 1.0 parts of vitamin E, and dissolve them in water to obtain a mixed solution.
[0058] (2) Add 1.0 parts of nosiheptide to the above mixed solution, and dissolve;
[0059] (3) spray drying.
[0060] The Nosiheptide soluble powder obtained in this example has a solubility of Nosiheptide in water of 2% at room temperature.
Embodiment 3
[0062] The carrier is selected from hydroxypropyl-β-cyclodextrin and polyamide-amine, the cosolvent is selected from poloxamer 188 and PVPK30, and the stabilizer is selected from vitamin C and L-cystine.
[0063] The preparation method of nosiheptide soluble powder is:
[0064] (1) Take 40 parts of hydroxypropyl-β-cyclodextrin, 28 parts of polyamide-amine, 17 parts of poloxamer 188, 13 parts of PVPK30, 1.0 parts of vitamin C, 1.0 parts of L-cystine, and dissolve in water to obtain a mixed solution.
[0065] (2) Add 1.5 parts of nosiheptide to the above mixed solution, and dissolve;
[0066] (3) spray drying.
[0067] The nosiheptide soluble powder obtained in this example has a solubility of nosiheptide in water of 2.1% at room temperature.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com