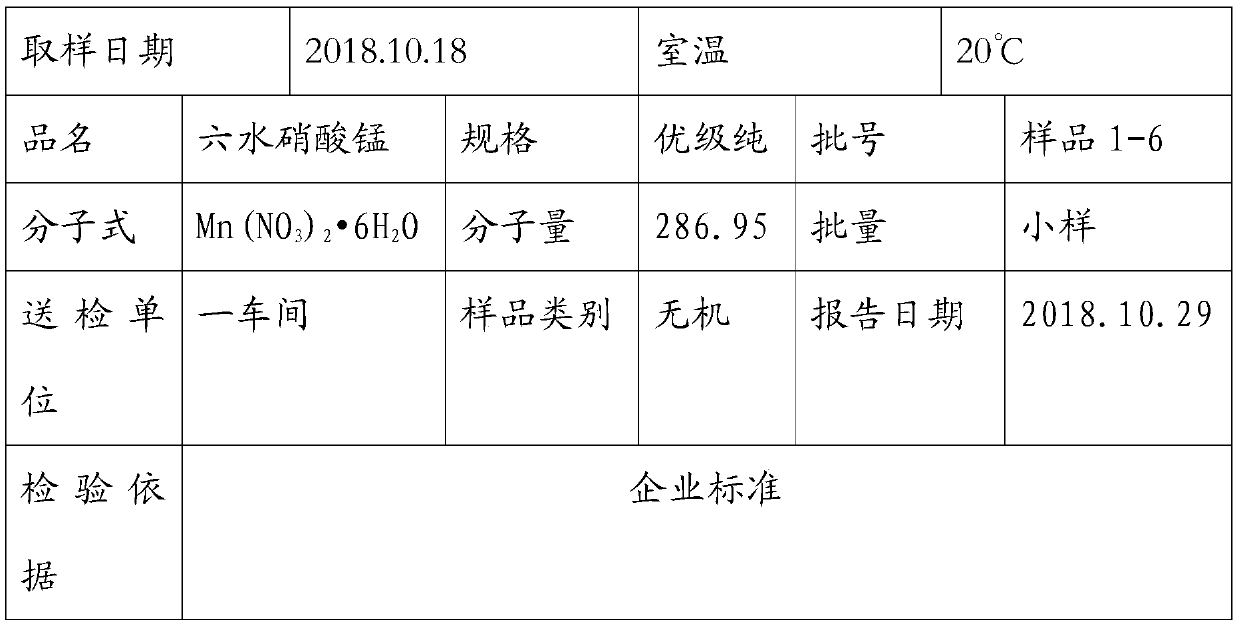
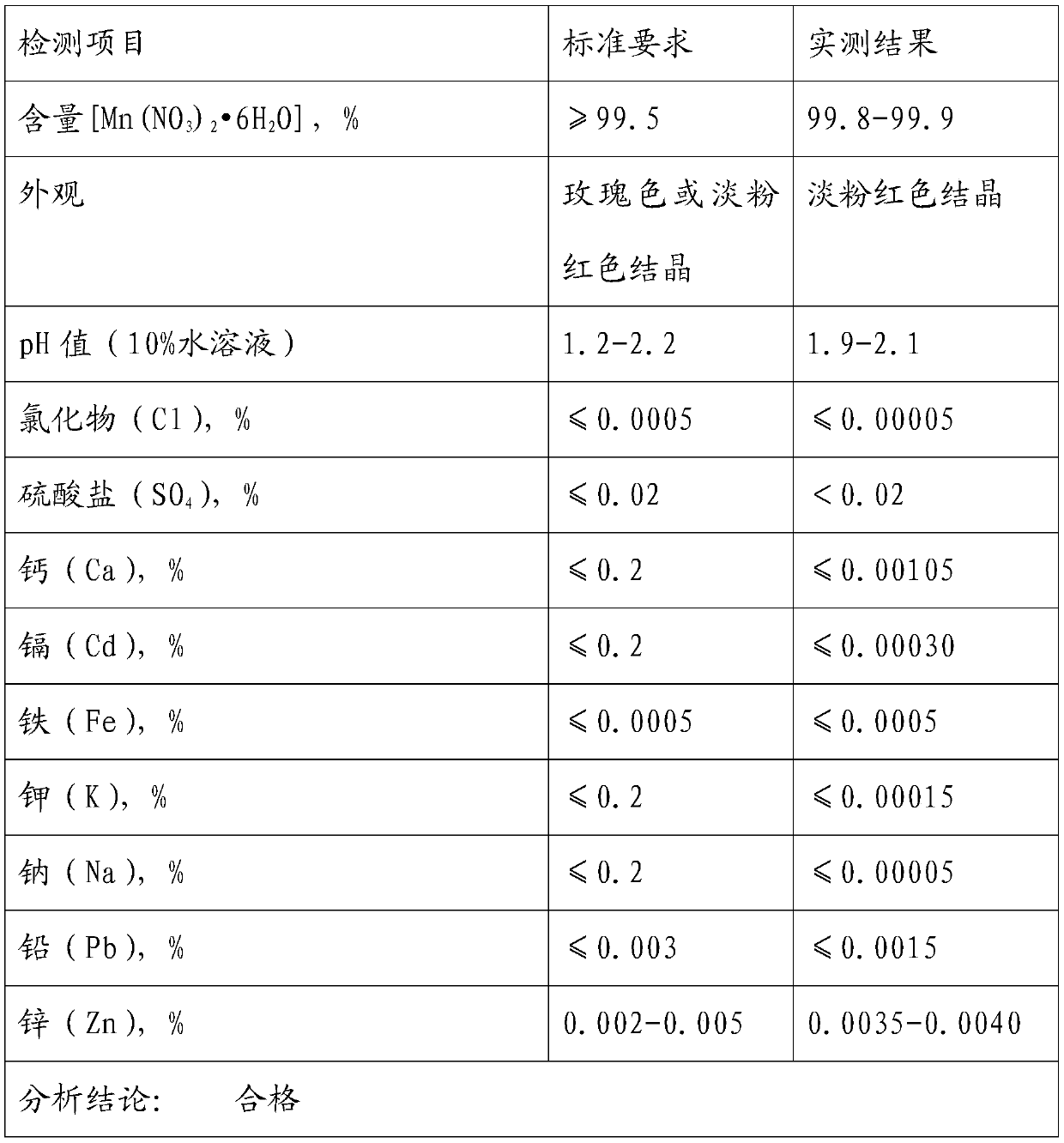
Method for producing high-purity manganese nitrate
A manganese nitrate, high-purity technology, applied in directions such as manganese nitrate, can solve the problems of yield impact, unfavorable low-cost production, poor impurity index, etc., and achieves the effect of low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A method for producing high-purity manganese nitrate, comprising the steps of:
[0023] Step 1. Put 3kg of electrolytic metal manganese in a closed 20L three-neck flask, and install a special quartz glass absorption device, then add 9kg of 70wt% industrial nitric acid, stir continuously during the addition process, and pass the yellow smoke generated during the synthesis into Gas scrubbing is carried out in the gas scrubbing device containing hydrogen peroxide to realize recycling, and the synthesis reaction time is controlled above 24 hours;
[0024] Step 2. When there is a trace amount of metal manganese in the solution that has not been reacted, it is judged that the synthesis reaction is completed. At this time, add 5g of ethylenediaminetetraacetic acid (EDTA) to the synthesis reaction solution, and keep stirring, then let it stand for precipitation , settling time 3h;
[0025] Step 3, after the standing precipitation is completed, the solution is filtered, then co...
Embodiment 2
[0028] A method for producing high-purity manganese nitrate, comprising the steps of:
[0029] Step 1. Put 3kg of electrolytic manganese metal in a closed 20L three-neck flask, and install a special quartz glass absorption device, then add 11kg of 60wt% industrial nitric acid, stir continuously during the addition process, and pass the yellow smoke generated during the synthesis into Gas scrubbing is carried out in the gas scrubbing device containing hydrogen peroxide to realize recycling, and the synthesis reaction time is controlled above 24 hours;
[0030] Step 2. When there is a trace amount of metal manganese in the solution that has not been reacted, it is judged that the synthesis reaction is completed. At this time, add 3g of ethylenediaminetetraacetic acid (EDTA) to the synthesis reaction solution, and keep stirring, then let it stand for precipitation , settling time 1h;
[0031] Step 3, after the standing precipitation is completed, the solution is filtered, then c...
Embodiment 3
[0034] A method for producing high-purity manganese nitrate, comprising the steps of:
[0035]Step 1. Put 3kg of electrolytic manganese metal in a closed 20L three-necked flask, and install a special quartz glass absorption device, then add 12kg of 60wt% industrial nitric acid, stir continuously during the addition process, and pass the yellow smoke generated during the synthesis into Gas scrubbing is carried out in the gas scrubbing device containing hydrogen peroxide to realize recycling, and the synthesis reaction time is controlled above 24 hours;
[0036] Step 2. When there is a trace amount of metal manganese in the solution that has not been reacted, it is judged that the synthesis reaction is completed. At this time, add 4g of ethylenediaminetetraacetic acid (EDTA) to the synthesis reaction solution, and keep stirring, then let it stand for precipitation , static precipitation time 2h;
[0037] Step 3, after the standing precipitation is completed, the solution is fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com