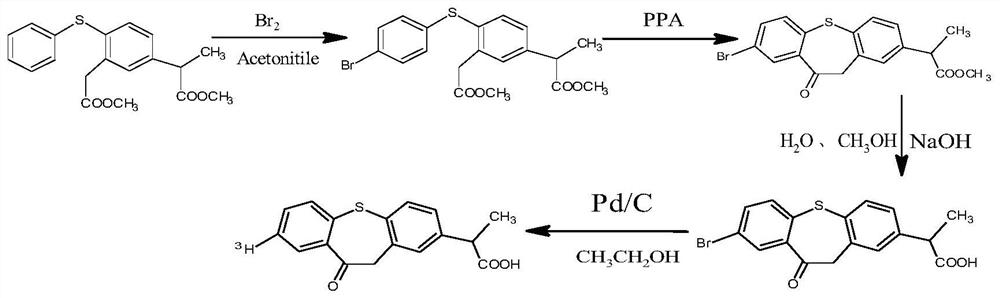
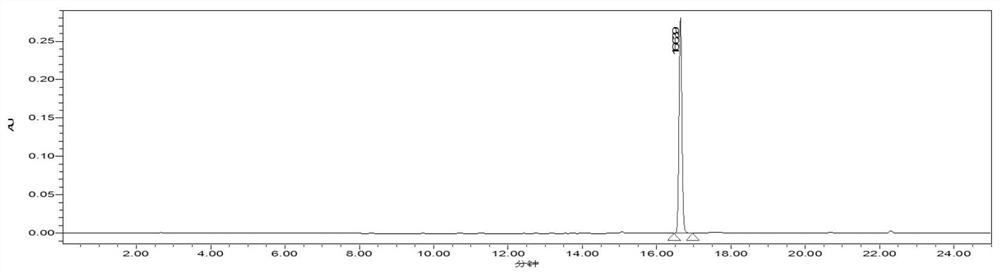
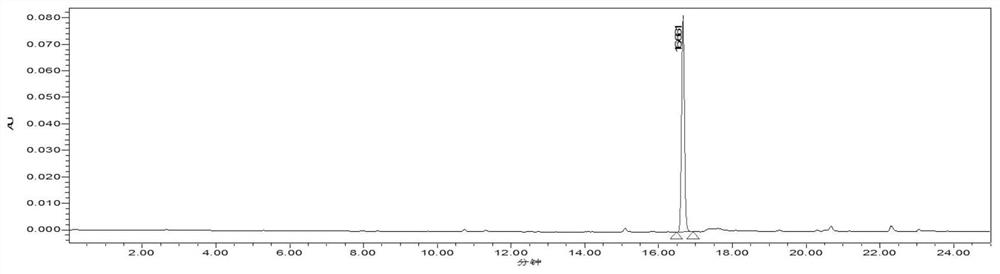
A kind of high specific activity tritiated zaltoprofen and its preparation method
An activity and labeling technology, applied in organic chemistry methods, chemical instruments and methods, introduction of heterocyclic compound isotopes, etc., to achieve good chemical stability and metabolic stability, high specific activity, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Take 5g of methyl 2-(3-methoxycarbonylmethyl-4-phenylthiophenyl)propionate, add 100mL of acetonitrile, after it dissolves, add 0.74mL of bromine, and react at 50°C for 10 hours , then distill off acetonitrile, add distilled water 100mL, extract with ethyl acetate (100mL×3), combine the ethyl acetate layers, distill off the solvent to obtain yellow oil 2-(3-methoxycarbonylmethyl-4-(4 -Bromophenylthio) phenyl) methyl propionate, yield 95%;
[0042] (2) Get 4g of methyl 2-(3-methoxycarbonylmethyl-4-(4-bromophenylthio)phenyl)propionate obtained in step (1), add 30g polyphosphoric acid (PPA), add Anhydrous sodium carbonate 0.5g, heat up to 98°C, stir and react for 10 hours, observe the formation of black viscous, stop the reaction, after cooling to room temperature, add water 100mL, ethyl acetate 200mL and stir for 10 minutes, let stand and separate , take the upper organic phase, evaporate the ethyl acetate to obtain a brown solid substance, which is recrystallized fro...
Embodiment 2
[0049] (1) Take 5g of methyl 2-(3-methoxycarbonylmethyl-4-phenylthiophenyl)propionate, add 120mL of acetonitrile, after it dissolves, add 0.74mL of bromine, and react at 35°C for 10 hours , then distill off acetonitrile, add distilled water 100mL, extract with ethyl acetate (100mL×3), combine the ethyl acetate layers, distill off the solvent to obtain yellow oil 2-(3-methoxycarbonylmethyl-4-(4 -Bromophenylthio) phenyl) methyl propionate, yield 94%;
[0050] (2) Get 4g of methyl 2-(3-methoxycarbonylmethyl-4-(4-bromophenylthio)phenyl)propionate obtained in step (1), add 25g polyphosphoric acid (PPA), add Anhydrous sodium carbonate 0.5g, heat up to 95°C, stir and react for 10 hours, observe the formation of black viscous, stop the reaction, after cooling to room temperature, add water 100mL, ethyl acetate 200mL and stir for 10 minutes, let stand and separate , take the upper organic phase, evaporate the ethyl acetate to obtain a brown solid substance, which is recrystallized fro...
Embodiment 3
[0057] (1) Take 5g of methyl 2-(3-methoxycarbonylmethyl-4-phenylthiophenyl)propionate, add 100mL of acetonitrile, after it dissolves, add 0.74mL of bromine, and react at 50°C for 10 hours , then distill off acetonitrile, add distilled water 100mL, extract with ethyl acetate (100mL×3), combine the ethyl acetate layers, distill off the solvent to obtain yellow oil 2-(3-methoxycarbonylmethyl-4-(4 -Bromophenylthio) phenyl) methyl propionate, yield 95%;
[0058] (2) Get 4g of methyl 2-(3-methoxycarbonylmethyl-4-(4-bromophenylthio)phenyl)propionate obtained in step (1), add 25g polyphosphoric acid (PPA), add Anhydrous sodium carbonate 0.5g, heat up to 98°C, stir and react for 8 hours, observe the formation of black viscous, stop the reaction, after cooling to room temperature, add water 100mL, ethyl acetate 200mL and stir for 10 minutes, let stand and separate , take the upper organic phase, evaporate the ethyl acetate to obtain a brown solid substance, which is recrystallized from...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com