Method for synthesizing boron (silicon) alkylated indole and tetrahydroquinoline through one-pot method
A technology for silylation of indole and tetrahydroquinoline, applied in chemical instruments and methods, organic chemistry, silicon organic compounds, etc., can solve the problems of harsh reaction conditions, low product yield, low atomic efficiency, etc. Easy to obtain, easy to operate, high tolerance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
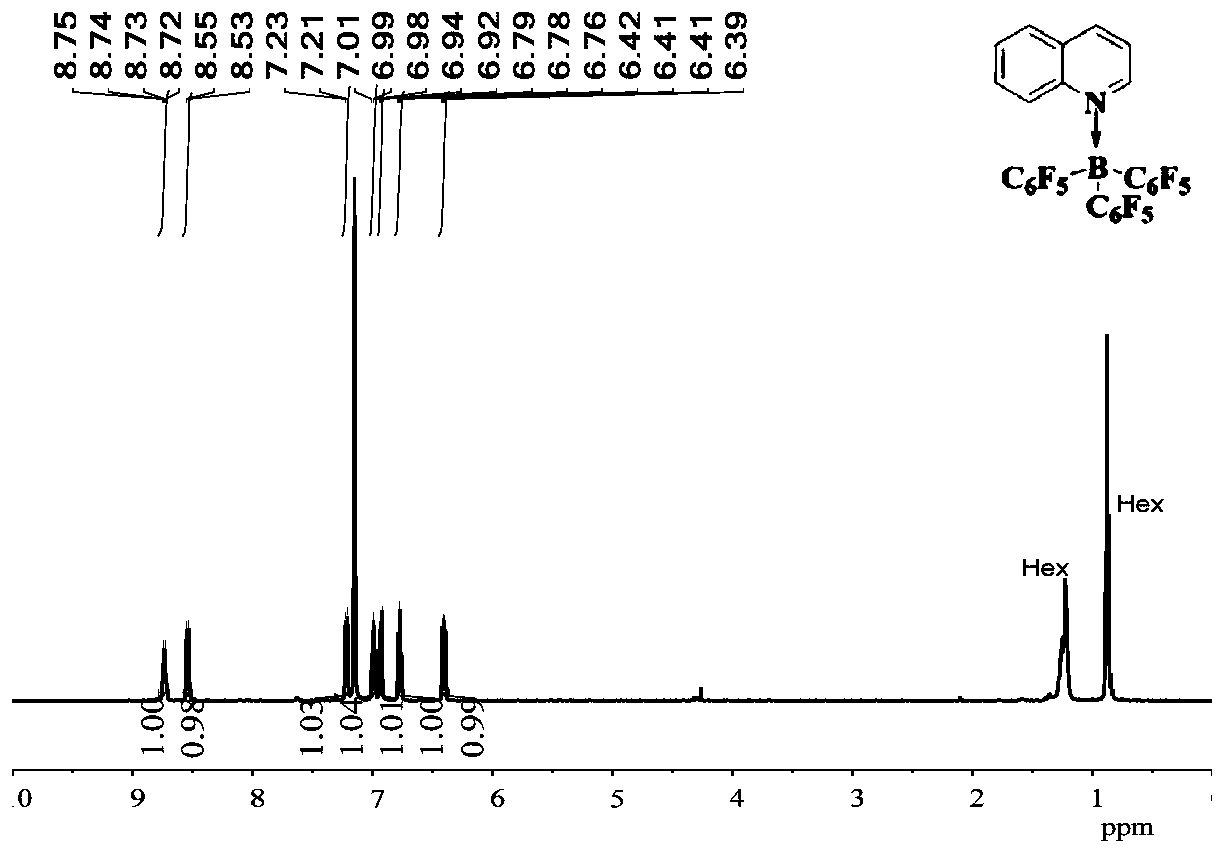
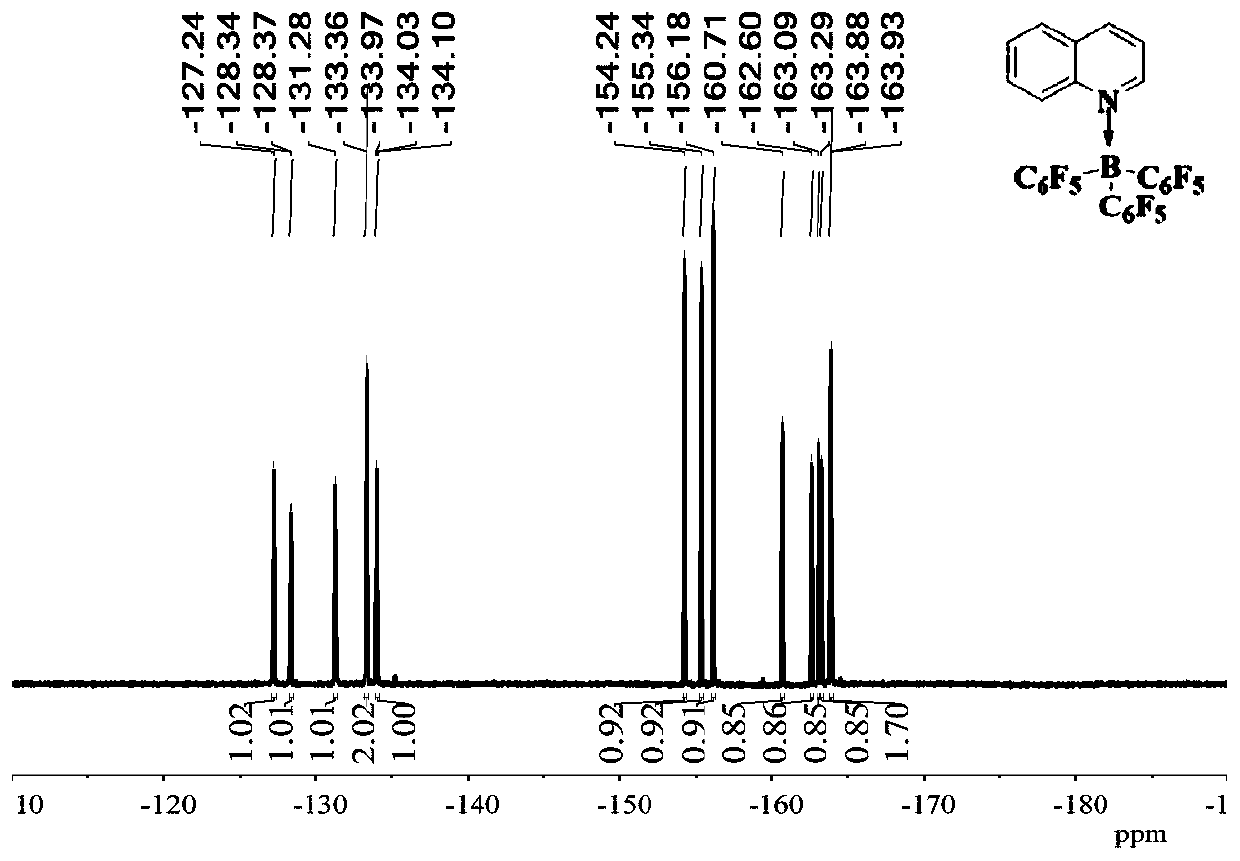
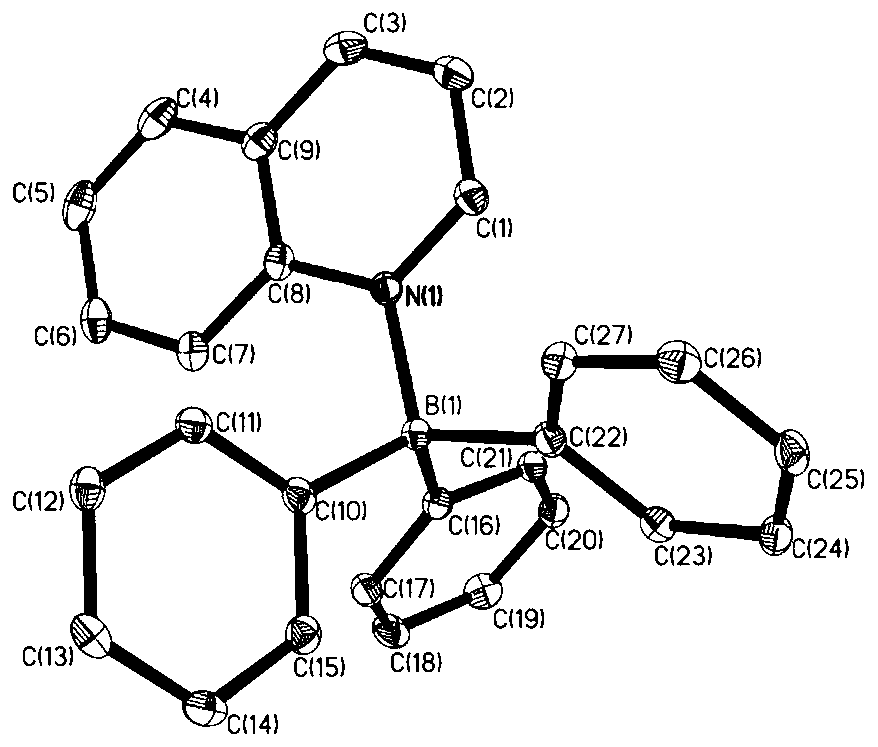
[0042] Embodiment 1 synthesizes and separates tripentafluorophenylborane and quinoline adduct B (C 6 f 5 ) 3 ·Quinoline(10)
[0043]
[0044] Weigh quinoline (2a, 25.8mg, 0.2mmol) in a vial in the glove box and dissolve it in 1mL of hexane, add B(C 6 f 5 ) 3 (4,102.4 mg, 0.2 mmol). The mixture was stirred rapidly at room temperature for 20 minutes, filtered, and the resulting solid was washed with hexane and dried in vacuo. A white solid B (C 6 f 5 ) 3 • Quinoline (10) (112.8 mg, 88% yield). ( 1 H / 19 See attached for F NMR chart figure 1 and 2 ) 1 H NMR (500MHz, C 6 D. 6 )δ:8.79–8.70(m,1H),8.54(d,J=9.0Hz,1H),7.22(d,J=8.0,1H),6.99(ddd,J=8.8,6.9,1.5Hz,1H) ,6.93(dd,J=8.30,1.5Hz,1H),6.80–6.74(m,1H),6.41(dd,J=8.0,5.8Hz,1H). 19 F NMR (471MHz,C 6 D. 6 )δ: -127.25 (t, J = 25.4Hz, 1F), -128.35 (q, J = 18.2, 15.4Hz, 1F), -131.28 (dt, J = 22.4, 10.8Hz, 1F), -133.14–- 133.52(m,2F),-134.03(ddd,J=30.8,24.0,8.8Hz,1F),-154.15–-154.34(m,1F),-155.34(t,J=20.9Hz,1F),-156....
Embodiment 2
[0045] Example 2 Synthesis and separation of catechol borane and quinoline adduct HBcat·Quinoline
[0046]
[0047] Quinoline (2a, 25.8 mg, 0.2 mmol) was weighed in a vial in the glove box and dissolved in 1 mL of hexane, and catecholborane (HBcat, 3, 24.0 mg, 0.2 mmol) was added thereto. The mixture was stirred rapidly at room temperature for 20 minutes, filtered, and the resulting solid was washed with hexane and dried in vacuo. HBcat·Quinoline (40 mg, yield 80%) was obtained as a yellow solid. ( 1 H / 13 C / 11 B NMR chart see attached Figure 4 , 5 、6) 1 H NMR (500MHz, C 6 D. 6 )δ: 9.01 (dd, J = 8.8Hz, 1H), 8.81 (dd, J = 5.0, 1.7Hz, 1H), 7.24 (ddd, J = 8.6, 6.9, 1.5Hz, 1H), 7.17–7.15 (m ,1H),7.15–7.13(m,2H),7.05(dd,J=8.2,1.5Hz,1H),6.97(ddd,J=8.0,6.8,1.1Hz,1H),6.87–6.81(m,2H ),6.28(dd,J=8.3,5.1Hz,1H),5.94–4.78(m,1H). 13 C{1H}(126MHz,C 6 D. 6 )δ: 151.3, 145.8, 142.2, 140.1, 131.2, 128.8, 127.8, 127.3, 125.2, 120.1, 120.0, 110.7. 11 B NMR (160MHz, C 6 D. 6 )δ...
Embodiment 3
[0048] Example 3 Gram-level Synthesis of 3-Borylated Indole and Tetrahydroquinoline
[0049]
[0050] In the glove box, weigh 1-methylindole (1.31g, 10mol), quinoline (1.29g, 10mmol), add in B (C 6 f 5 ) 3 in benzene solution (25mL, 0.5mmol). In a 50mL reaction flask, stir well and add catecholborane (2.4g, 20mmol). After stirring at room temperature for 2 h, concentrate, add 20 mL of hexane, stir for 10 min, and filter to obtain a white solid. After washing and drying, 1.87 g of 3-position borated indole was obtained, with a yield of 75%. ( 1 H / 13 See attached for C NMR chart Figure 7 , 8 ) 1 H NMR (500MHz, C 6 D. 6 )δ: 8.59(d, J=7.9Hz, 1H, HAr), 7.37-7.32(m, 2H, HAr), 7.25(t, J=7.3Hz, 1H, HAr), 7.19(m, 2H, HAr) ,6.98(d,J=8.1Hz,1H,HAr),6.88-6.83(m,2H,HAr),2.79(s,3H,NCH3). 13 C{ 1 H}NMR (126MHz,C 6 D. 6 )δ: 148.8, 139.3, 138.1, 132.5, 128.0, 122.7, 122.3, 122.2, 121.1, 112.1, 110.0, 31.8.. The mother liquor was collected, added 5 mL of methanol for hydrolys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com