Preparation method of imidazolium salt antibacterial polymer
A technology of polymers and salts, applied in botany equipment and methods, chemicals for biological control, animal repellants, etc., can solve negative effects, silver wound dressings are expensive, worry about nanoparticles absorbing nanoparticles Toxicity and other issues, to achieve good biocompatibility, low cytotoxicity, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
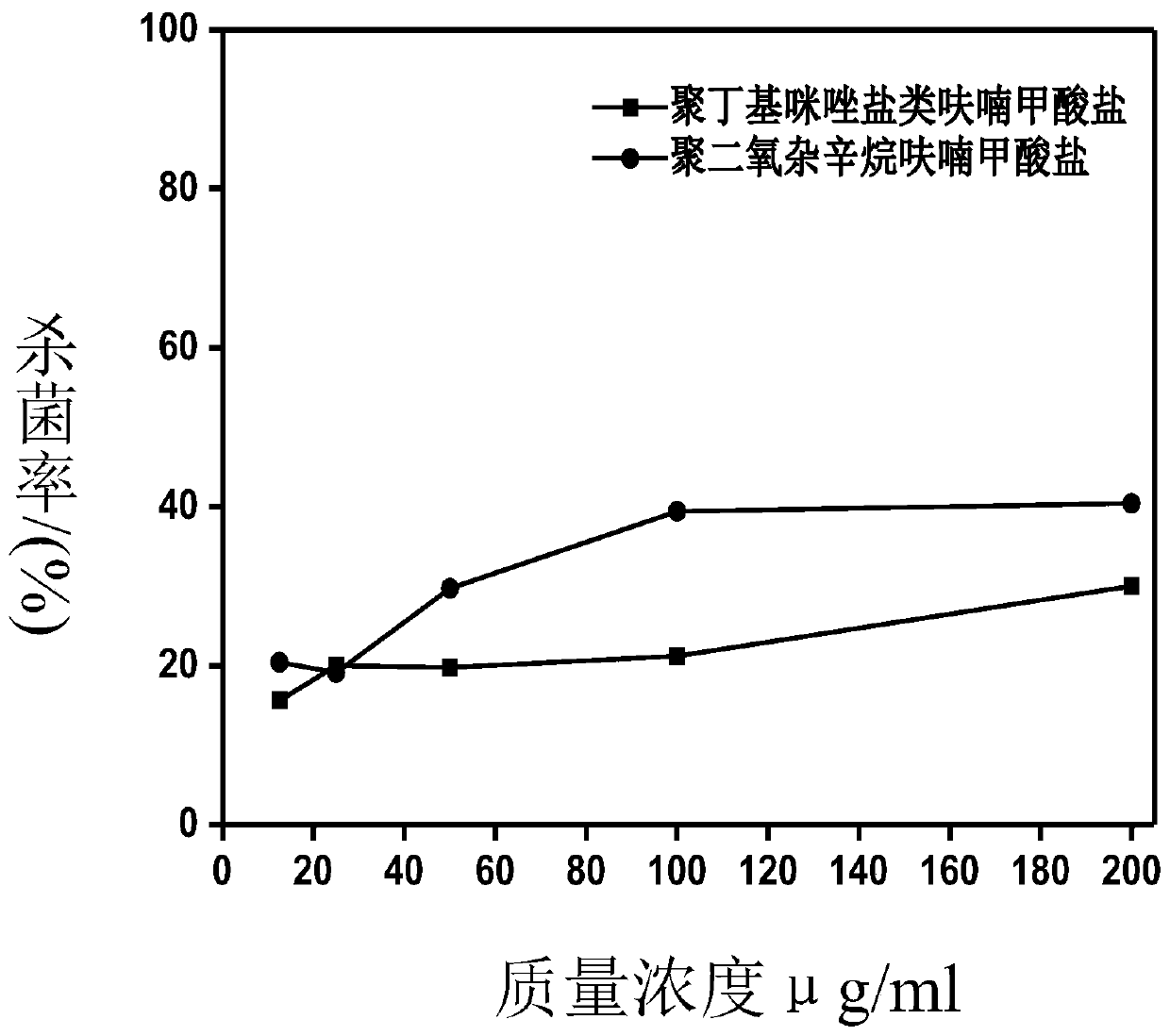
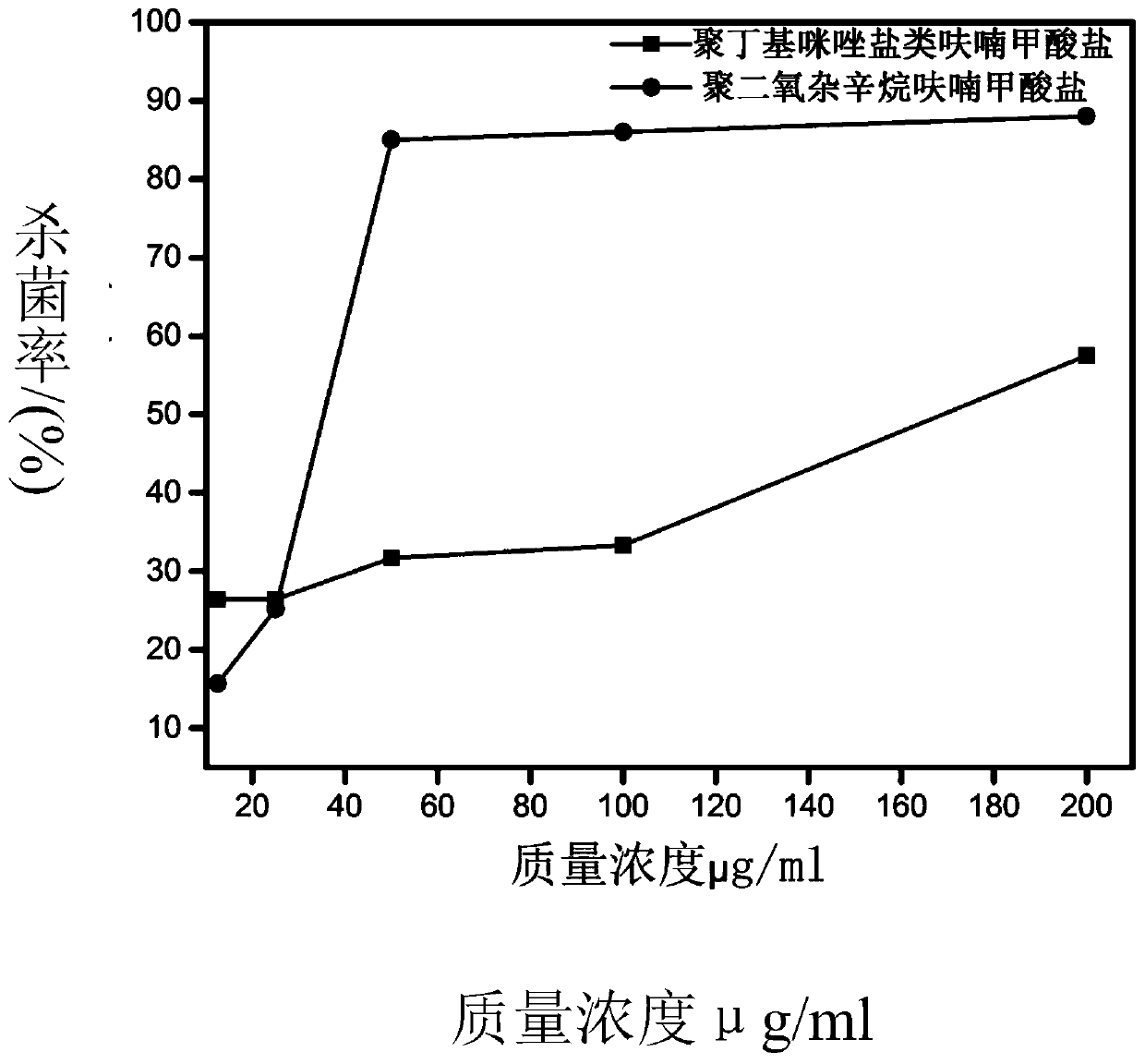
Image
Examples
Embodiment 1
[0021] Add 2.25ml of 1,4-butanediamine into a round bottom flask, dissolve it with 11.25ml of deionized water, and react at room temperature for 1h. Subsequently, under an ice bath at 0°C, 7.5 g of furoic acid was added, and after it was completely dissolved, 2.55 ml of glyoxal and 1.58 ml of formaldehyde were added respectively. Then use a pH meter to measure the pH value of the solution at this time, and stabilize it at about 7 by adding NaOH solution or HCL solution dropwise. After the adjustment, the temperature of the oil bath was raised to 100° C., and the reaction was continued for 36 hours. After the reaction was completed, the solution was transferred to a dialysis bag and dialyzed for 24 hours to remove unreacted raw materials. After the dialysis, the solution was put into a -80°C refrigerator for freezing, and then put into a freeze dryer for freeze-drying to obtain a dark yellow imidazolium salt antibacterial polymer.
Embodiment 2
[0023] Add 3.95ml of 1,6-hexanediamine into a round bottom flask, dissolve it with 19.75ml of deionized water, and react at room temperature for 1h. Subsequently, 8.3 g of furoic acid was added under an ice bath at 0° C., and after it was completely dissolved, 2.55 ml of glyoxal and 1.58 ml of formaldehyde were added respectively. Then use a pH meter to measure the pH value of the solution at this time, and stabilize it at about 7 by adding NaOH solution or HCL solution dropwise. After the adjustment, the temperature of the oil bath was raised to 100° C., and the reaction was continued for 36 hours. After the reaction was completed, the solution was transferred to a dialysis bag and dialyzed for 24 hours to remove unreacted raw materials. After the dialysis, the solution was put into a -80°C refrigerator for freezing, and then put into a freeze dryer for freeze-drying to obtain a dark yellow imidazolium salt antibacterial polymer.
Embodiment 3
[0025] Add 4.41ml of 1,8-octanediamine into the round bottom flask, dissolve it with 22ml of deionized water, and react at room temperature for 1h. Subsequently, 6.9 g of furoic acid was added under an ice bath at 0° C., and after it was completely dissolved, 2.55 ml of glyoxal and 1.58 ml of formaldehyde were added respectively. Then use a pH meter to measure the pH value of the solution at this time, and stabilize it at about 7 by adding NaOH solution or HCL solution dropwise. After the adjustment, the temperature of the oil bath was raised to 100° C., and the reaction was continued for 36 hours. After the reaction was completed, the solution was transferred to a dialysis bag and dialyzed for 24 hours to remove unreacted raw materials. After the dialysis, the solution was put into a -80°C refrigerator for freezing, and then put into a freeze dryer for freeze-drying to obtain a dark yellow imidazolium salt antibacterial polymer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com