Application of a recombinant esterase, gene, engineering bacteria and resolution of (r,s)-indoline-2-carboxylate ethyl ester
A technology of recombinant esterase and indoline, applied in the direction of genetic engineering, application, plant gene improvement, etc., can solve the problems of expensive, serious pollution, split reagent price, and many steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: recombinant esterase BaCE
[0060] Bacillus B8W22 (Bacillus aryabhattai), preserved in the China Center for Type Culture Collection (address China, Wuhan, Wuhan University, 430072), preservation number CCTCC NO:M 2017751, preservation date November 30, 2017, has previously applied for a patent (Application No.: 2019101300148) submitted relevant strain preservation information and disclosed the source of its genetic resources.
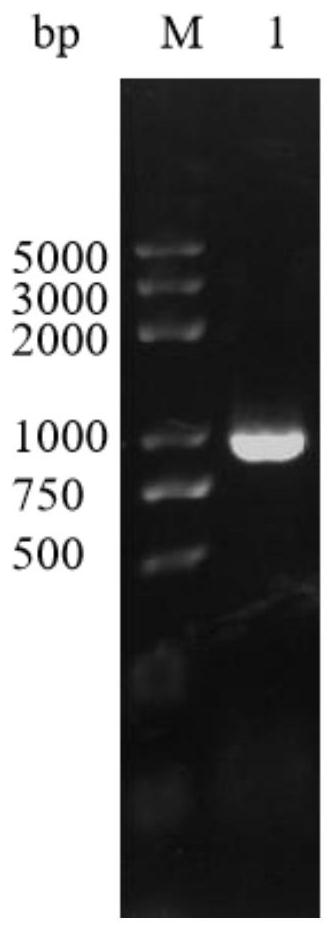
[0061]1. Extract the genomic DNA of Bacillus CCTCC NO:M 2017751 with a nucleic acid extraction kit, use the genomic DNA as a template, and use primer pET-28a-noc1-F (5'-TAAGAAGGAGATATACCATGGATGCCGTTAGATCCGCATATTC-3'), primer pET-28a- xho1-R(5'-GTGGTGGTGGTGGTGCTCGAGAATGGAGTCAAAT
[0062] ACTTGACGCAG-3') under the effect of PCR amplification.
[0063] The amount of each component in the PCR reaction system (total volume 20 μL):
[0064] Primer pET-28a-nco1-F and primer pET-28a-xho1-R each 0.3 μL, genomic DNA 1 μL, primesTARMax Prem...
Embodiment 2
[0071] Embodiment 2: recombinant esterase BaCE bacterial cell
[0072] The recombinant Escherichia coli BL21 / pET-28a-BaCE obtained according to Example 1 was inoculated into LB liquid medium containing 100 μg / ml kanamycin, cultivated at 37°C for 12-16h, and then inoculated at 1% (v / v) Inoculate it into fresh LB liquid medium containing 100 μg / ml kanamycin, and cultivate it at 37°C until the cell concentration is OD 600 0.6, then add IPTG with a final concentration of 0.2mM to the LB liquid medium, induce culture at 22°C for 10-12h, then centrifuge at 8000rpm at 4°C for 10min, collect the wet cells, which are cells containing recombinant esterase .
Embodiment 3
[0074] 1 g of recombinant Escherichia coli BL21 / pET-28a-BaCE wet thallus obtained in Example 2 was resuspended with 20 ml, 50 mM Tris-HCl buffer solution of pH 8.0, and then ultrasonicated (ultrasonic power was 250 W, ultrasonic 2 s, Interval 6s, effective ultrasonic time 10min), the broken suspension was centrifuged at 12000rpm at 4°C for 10min, cell debris was discarded as much as possible, and the supernatant was taken to obtain protein crude enzyme solution. According to the instructions of Ni-NTA metal chelate affinity chromatography, take the protein crude enzyme solution and load it on the pre-equilibrated Ni 2+ In the column, the impurity protein and the target protein were eluted successively with an aqueous solution containing 50 mM imidazole aqueous solution, 100 mM imidazole aqueous solution, 150 mM imidazole aqueous solution, and 200 mM imidazole aqueous solution. Collect the effluent after elution with 100mM imidazole aqueous solution, ultrafiltration membrane (1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com