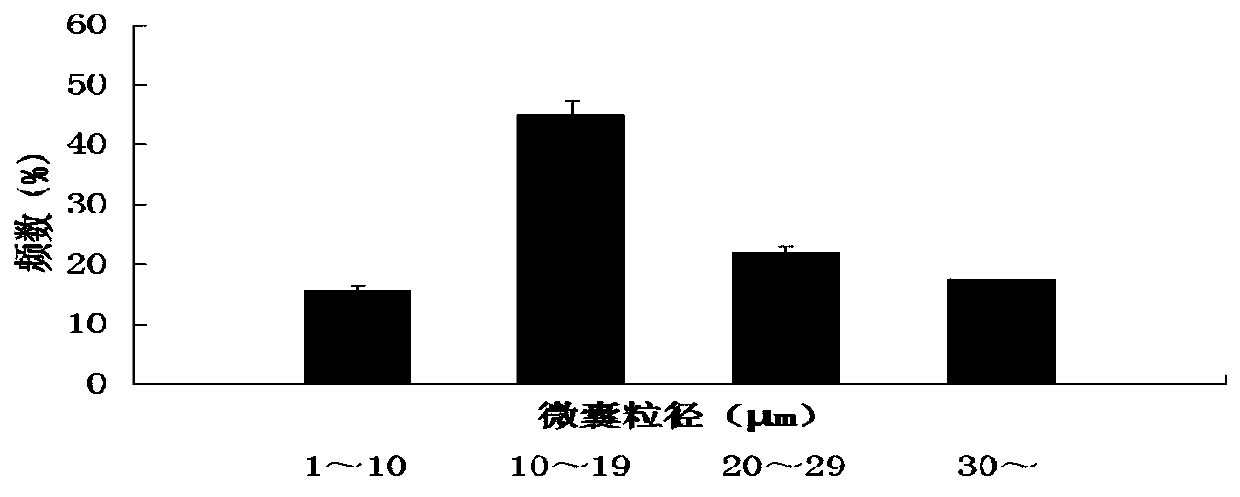
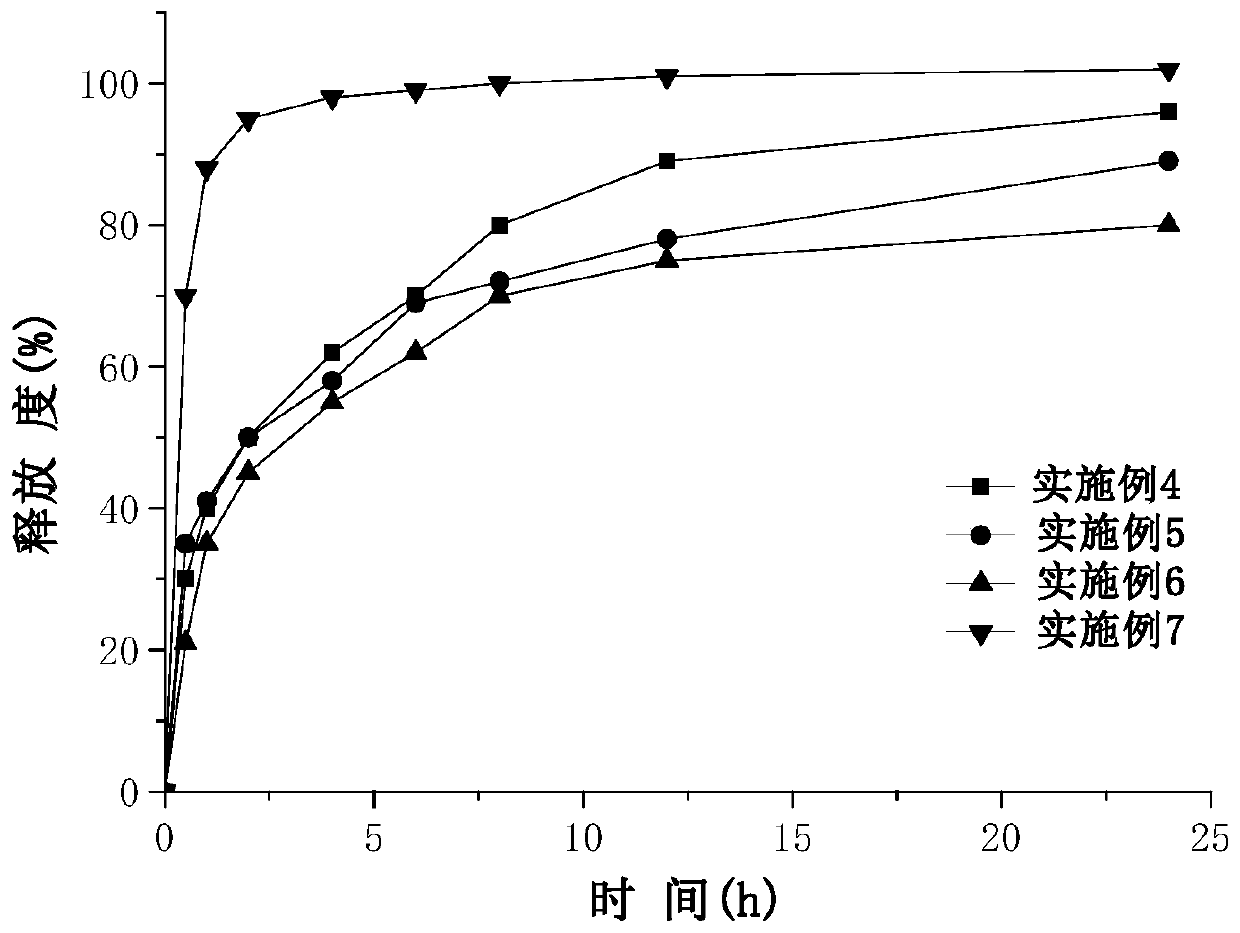
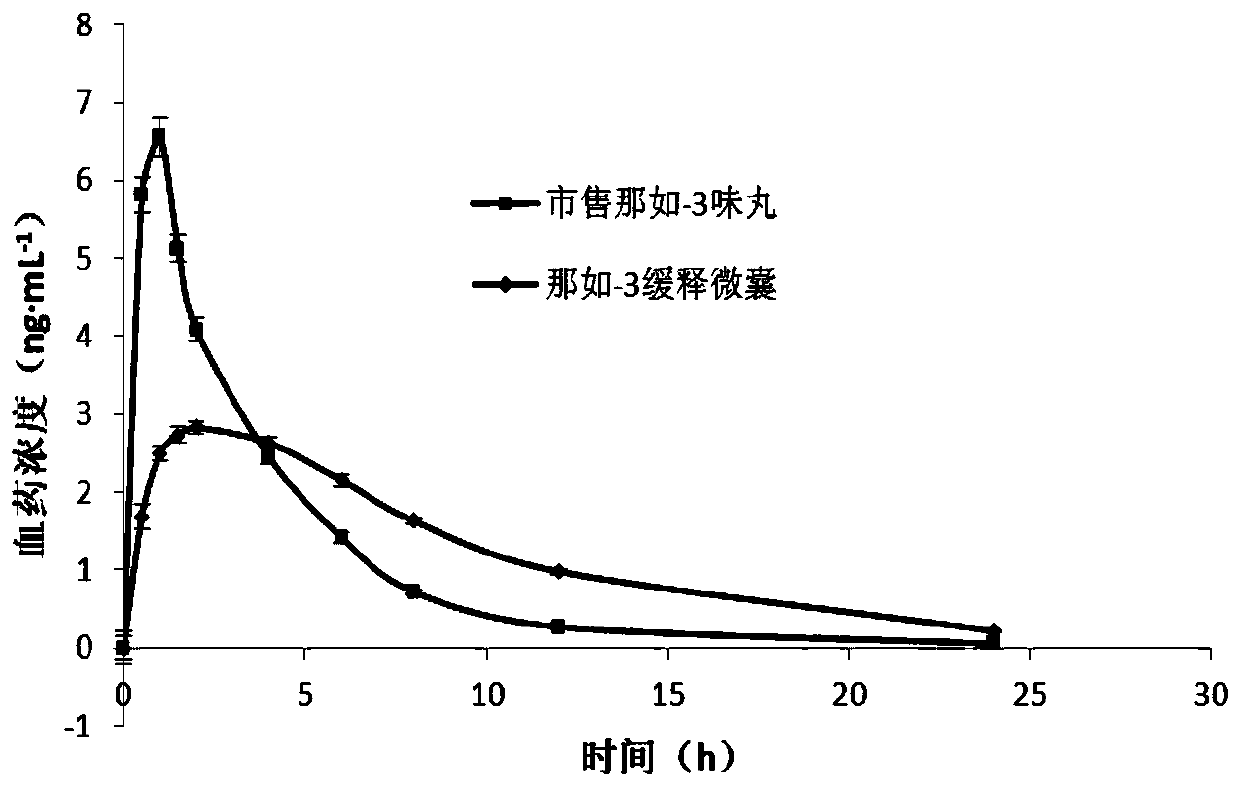
Naru-3 sustained-release micro-capsule sphere and preparation method thereof
A small ball, slow-release technology, applied in microcapsules, pharmaceutical formulations, capsule delivery, etc., can solve the problem of not being able to fundamentally control the drug release rate, achieve individualized drug administration, simple and fast preparation methods, and improve drug intake. compliance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]A prescription for Naru-3 sustained-release microcapsules, the components are expressed in mass fractions, including 20% of capsule core Naru-3 extract powder, 60% of polymer capsule material ethyl cellulose, plasticized and pore-forming Agent polyvinylpyrrolidone 18%, anti-sticking agent micro-powder silica gel 2%;
[0033] Among them, the ethyl cellulose is ethyl cellulose N20 produced by Guangzhou Daojun Biotechnology Co., Ltd.; the polyvinylpyrrolidone is povidone k30 produced by Dongying Huaan Chemical Co., Ltd. Polyvinylpyrrolidone and micropowder silica gel are produced by Tianjin Yongsheng Fine Chemical Co., Ltd.
Embodiment 2
[0035] A prescription for Naru-3 sustained-release microcapsules, the components are expressed in mass fractions, including 22% of capsule core Naru-3 extract powder, 54% of polymer capsule material ethyl cellulose, plasticized and pore-forming Agent glycerin 22%, anti-tack agent talcum powder 2%;
[0036] Ethyl cellulose is the ethyl cellulose of ethyl cellulose N100 that the model produced by Guangzhou Daojun Biotechnology Co., Ltd.; Talcum powder is produced by Tianjin Damao Chemical Reagent Factory.
Embodiment 3
[0038] A Naru-3 sustained-release microcapsule prescription, the components are in mass fraction, including 18% of the capsule core Naru-3 extract powder, 54% of polymer capsule material acrylic resin, plasticizing porogen poly Ethylene glycol 27%, anti-sticking agent micro-powder silica gel 2%;
[0039] Wherein, acrylic resin is the acrylic resin of Eudragit RL100 produced by Colorcon Company; Polyethylene Glycol is the Polyethylene Glycol of PEG6000 produced by Tianjin Fuchen Chemical Reagent Factory.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap