Method for preparing single crystal or amorphous substance of medicine or medicine intermediatemedicinemedicine with adjustable particle size
An amorphous and intermediate technology, applied in the direction of single crystal growth, single crystal growth, chemical instruments and methods, etc., can solve the problems of poor controllability of crystal nucleation and growth, easy generation of polycrystals or twins, etc. Solve the effect that the formation of single crystal is not easy to control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0154] According to an embodiment of the present invention, the method for cultivating a single crystal further includes the following steps:
[0155] (b1) transferring the single crystal of the drug or drug intermediate prepared above to the mother liquor of the drug or drug intermediate for cultivation;
[0156] (b2) Collecting the single crystal in step (b1).
[0157] According to an embodiment of the present invention, the transfer is any method known to those skilled in the art that can remove a single crystal, including but not limited to optical microscope transfer, scanning electron microscope transfer, dual-beam electron microscope transfer , Transmission electron microscope transfer in one or a combination of several.
[0158] According to an embodiment of the present invention, the mother liquor is a mother liquor system known by those skilled in the art and compatible with the single crystal phase to be cultivated, for example, it can be a saturated solution syste...
Embodiment 1
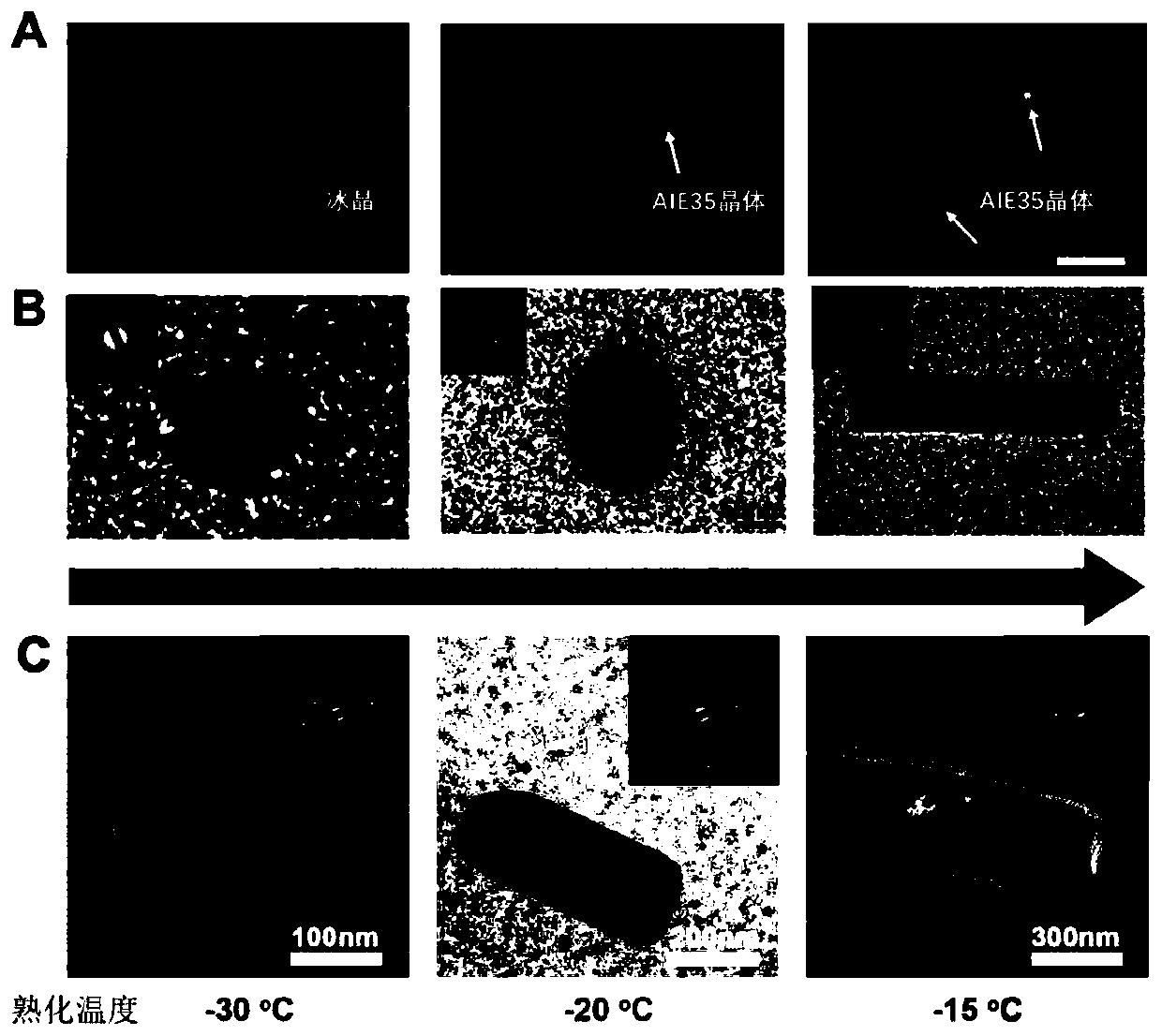
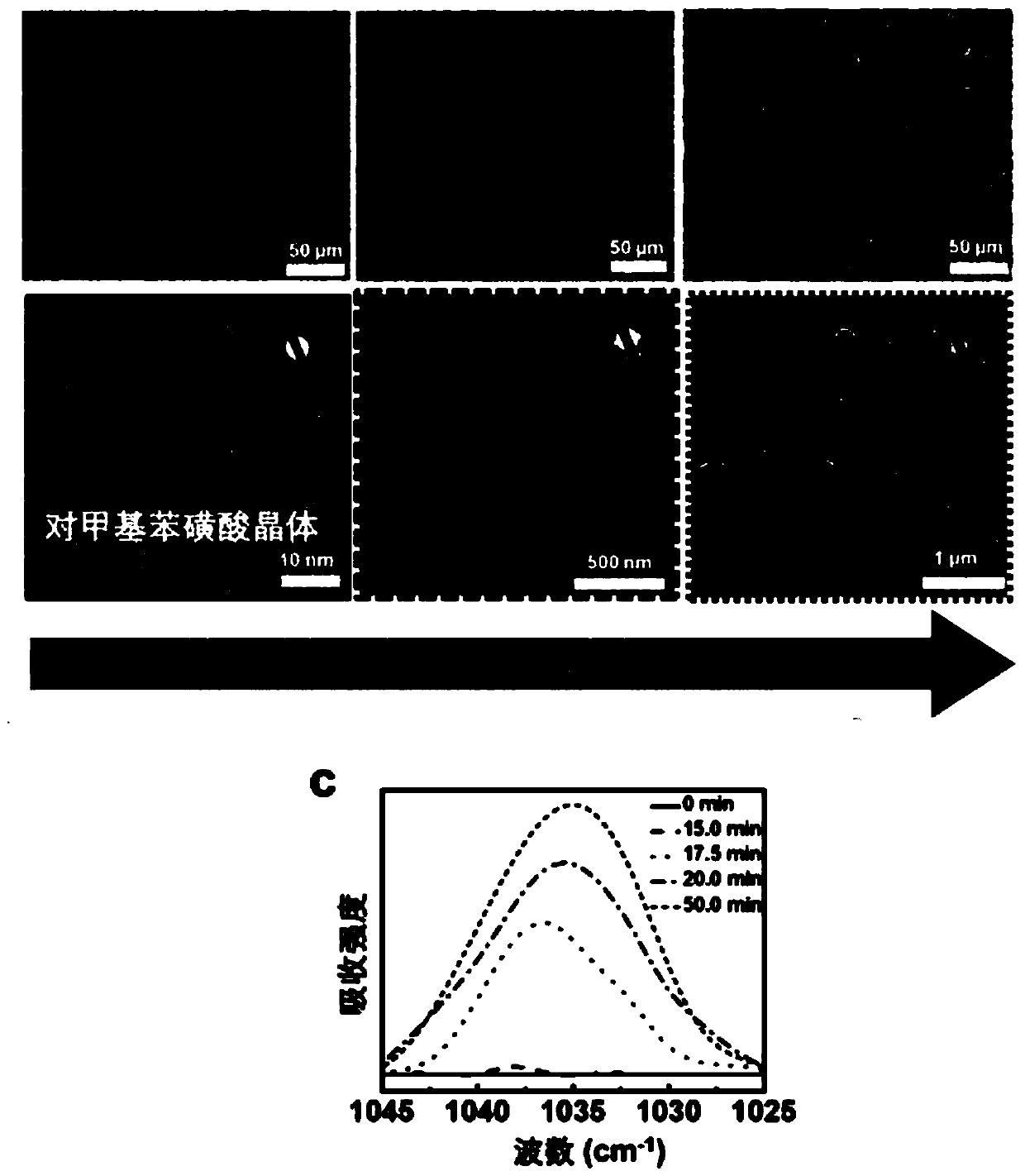
[0162] Prepare a 10-hydroxycamptothecin solution with a concentration of 5 μM in dimethyl sulfoxide, take 5 parts of 5 mL of the solution into a beaker with a graduated cylinder, and place it in liquid nitrogen at -196 ° C until it is completely frozen, and then Slowly heat up at 8°C / min and place them in temperature-controlled cold traps at -50°C, -45°C, -40°C, -35°C, and -25°C for aging for 40 minutes, and then freeze-dry the sample to completely sublimate the solid dimethylformamide 10-Hydroxycamptothecin single crystal nanoparticles can be obtained, and the average particle size is continuously adjustable from 10nm to 1000nm. The test results are shown in Figure 4 ,from Figure 4 It can be seen from the figure that the average particle diameters of the prepared 10-hydroxycamptothecin single crystal nanoparticles are 10.7nm, 130nm, 420nm, 650nm, 1190nm, respectively. Finally, 1000 mL of 0.1 mg / mL Tween-80 solution was used to collect the 10-hydroxycamptothecin single crys...
Embodiment 2
[0165] Use 95wt% chloroform-5wt% methanol mixed solvent to prepare a 10-hydroxycamptothecin solution with a concentration of 5mM, take 100μL of the solution with a syringe, spread it on a silicon wafer, and place it in -196°C liquid nitrogen to cool down completely Freeze, then slowly heat up at 5°C / min and place them in temperature-controlled cold traps at -100°C, -96°C, -88°C, -85°C, and -79°C for 45 minutes, then freeze-dry the sample and sublime completely solid solvent, 10-hydroxycamptothecin single crystal nanoparticles can be obtained, and the average particle sizes are 12nm, 105nm, 440nm, 680nm, 1200nm respectively. Finally, 1000 mL of 0.1 mg / mL Span-80 solution was used to collect the obtained single crystal nanoparticles to form a stable suspension.
[0166] In addition, spread 50 μL of the above-mentioned 5 mM 10-hydroxycamptothecin solution on the silicon wafer cooled to -150 °C, and then raise the temperature to -100 °C, -88 °C, -79 °C at a heating rate of 25 °C / m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com