Flavone synthase and application thereof
A flavonoid and enzyme activity technology, applied in the field of biological enzymes, can solve the problem that the conversion efficiency of FNSI cannot meet the requirements, and achieve the effect of optimal catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1. Cloning of flavone synthase DcFNS and its expression in Escherichia coli
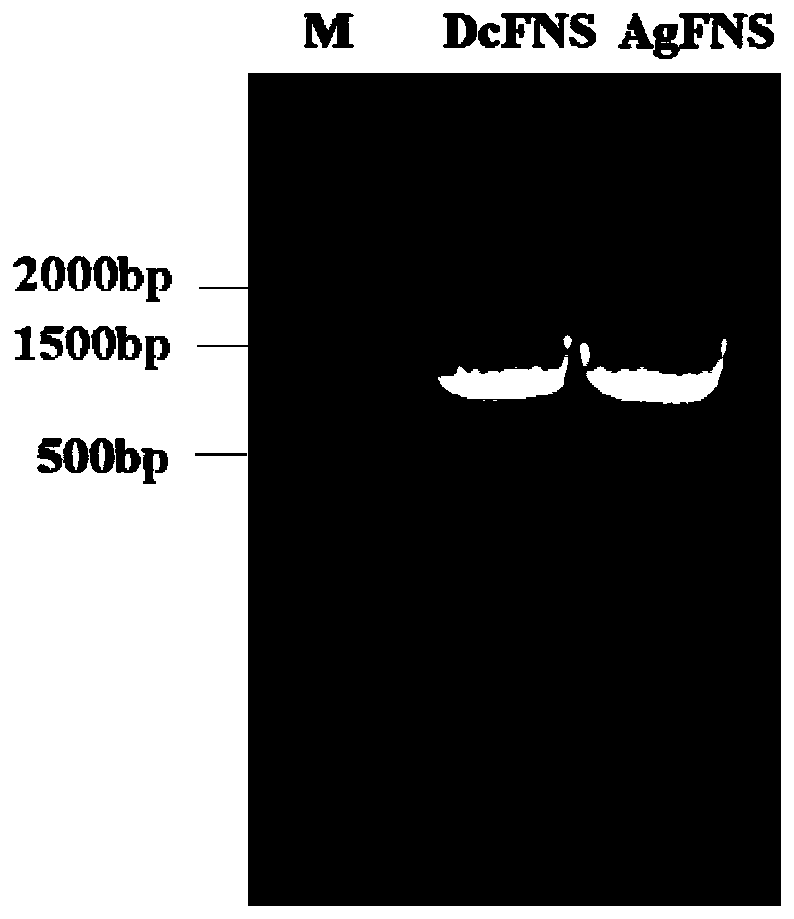
[0025] Two primers such as SEQ ID NO:5 and SEQ ID NO:6 in the sequence listing were synthesized, and the cDNA obtained by reverse transcription of RNA extracted from plants was used as a template to perform PCR using the above primers. The DNA polymerase was selected from the high-fidelity KOD DNA polymerase of Treasure Bioengineering Co., Ltd. The PCR amplification program was as follows: 94°C for 2min; 94°C for 15s, 58°C for 30s, 68°C for 2min, a total of 35 cycles; 68°C for 10min to 10°C. The PCR products were detected by agarose gel electrophoresis, and the results are shown in the attached figure 1 .
[0026] Under UV irradiation, the target DNA band is excised. Then Axygen Gel Extraction Kit (AEYGEN Company) was used to recover DNA from the agarose gel, which was the amplified DNA fragment of the flavone synthase gene. The recovered PCR product was cloned into the PMDT vector...
Embodiment 2
[0031] Embodiment 2. Flavone synthase DcFNS catalyzes naringenin reaction
[0032] Using the supernatant of BL21-pET28a-DcFNS and BL21-pET28a lysate obtained in Example 1 as the crude enzyme solution, configure the following reaction system (100 μL):
[0033]
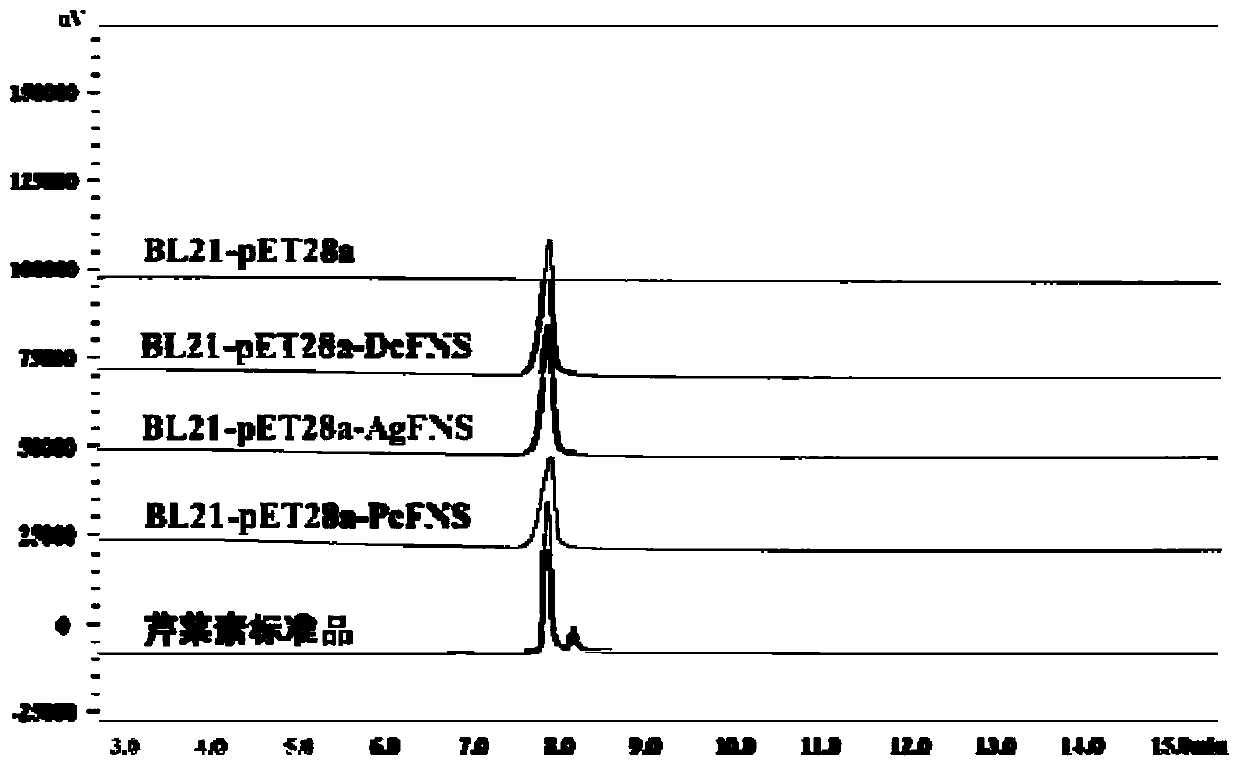
[0034] React in a water bath at 30°C for 2h. After the reaction was completed, an equal volume of ethyl acetate was added for extraction, and the upper ethyl acetate phase was taken. After vacuum concentration, the reaction product was dissolved in 100 μL of methanol, and the results were detected by HPLC. The results are shown in the attached image 3 .
[0035] from image 3 From the results, it can be seen that the Escherichia coli crude enzyme solution BL21-pET28a-DcFNS containing flavone synthase DcFNS can catalyze naringenin to form a new product, and its retention time in HPLC is consistent with that of the standard apigenin, while the control group contains empty The Escherichia coli crude enzyme solution ...
Embodiment 3
[0036] Example 3. Cloning of flavone synthase AgFNS and its expression in Escherichia coli
[0037] Two primers such as SEQ ID NO: 9 and SEQ ID NO: 10 in the sequence listing were synthesized, and the cDNA obtained by reverse transcription of RNA extracted from plants was used as a template to perform PCR using the above primers. The DNA polymerase was selected from the high-fidelity KOD DNA polymerase of Treasure Bioengineering Co., Ltd. The PCR amplification program was as follows: 94°C for 2min; 94°C for 15s, 58°C for 30s, 68°C for 2min, a total of 35 cycles; 68°C for 10min to 10°C. The PCR products were detected by agarose gel electrophoresis, and the results are shown in the attached figure 1 .
[0038] Under UV irradiation, the target DNA band is excised. Then Axygen Gel Extraction Kit (AEYGEN Company) was used to recover DNA from the agarose gel, which was the DNA fragment of the amplified flavone synthase gene. The recovered PCR product was cloned into a PMDT vecto...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com