Tetragenus active bacterial agent for treating diarrhoea and application of tetragenus active bacterial agent
A live bacteria preparation and diarrhea technology, applied in the field of medicine, can solve problems such as uneven products, dog intestinal flora imbalance, and no microecological preparations for dogs, etc., to inhibit pathogenic bacteria, improve body immunity, improve The effect of gut microbiota structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] A quadruple active bacterial preparation for treating diarrhea, comprising 5% probiotic mixture, 6% xylooligosaccharide, and the balance being glucose;
[0015] The probiotic mixture includes freeze-dried powder of Bifidobacterium animalis, freeze-dried powder of Lactobacillus paracasei, freeze-dried powder of Enterococcus faecium and freeze-dried powder of Saccharomyces cerevisiae.
[0016] The live bacteria ratio of Bifidobacterium animalis, Lactobacillus paracasei, Enterococcus faecium and Saccharomyces cerevisiae is 10:10:1:1.
[0017] The number of live bacteria of Bifidobacterium animalis B1014 strain ≥ 2.0×10 9 CFU / g, live bacteria count of Lactobacillus paracasei L1056 strain ≥2.0×10 9 CFU / g, the number of live bacteria of Enterococcus faecium EF1012 strain ≥ 2.0×10 8 CFU / g, the number of live bacteria of Saccharomyces cerevisiae S1072 strain ≥2.0×10 8 CFU / g.
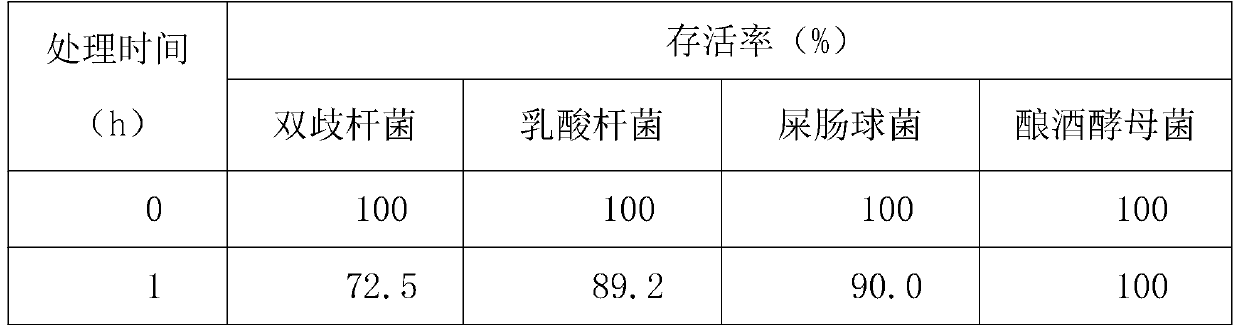
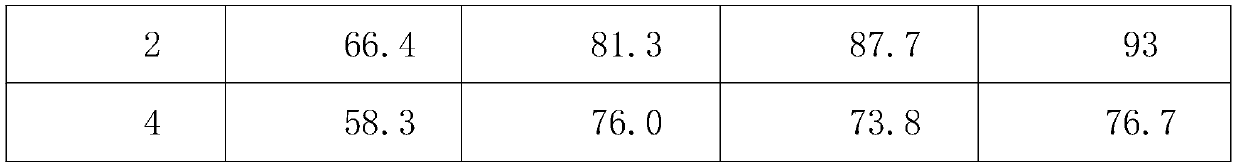
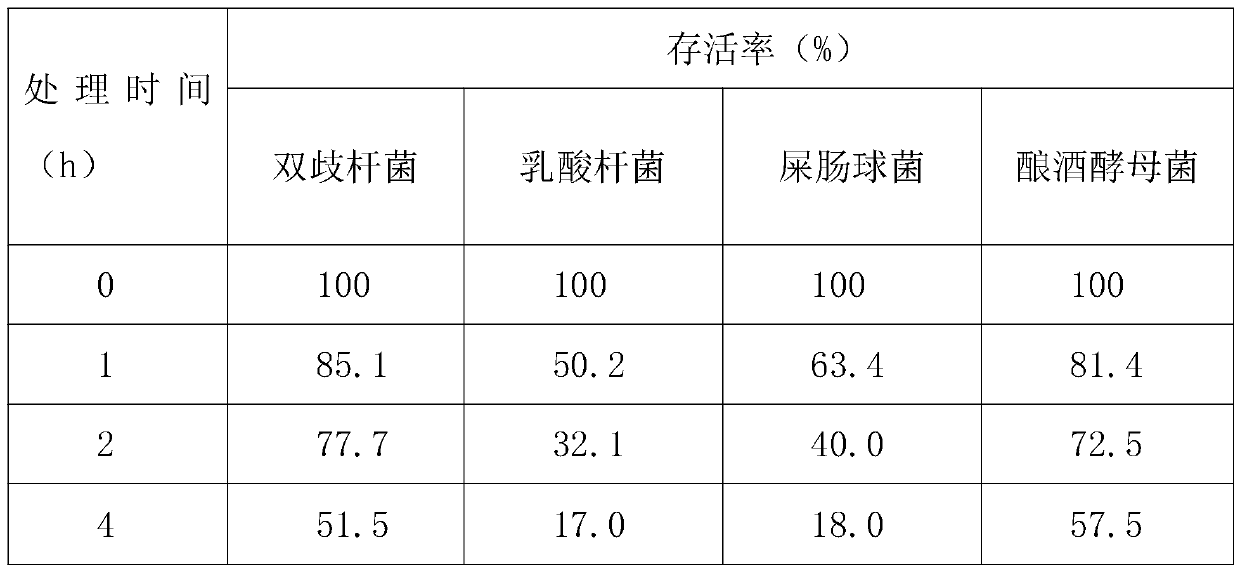
[0018] The survival rate of Lactobacillus paracasei, Enterococcus faecium and Saccharomyces cerevi...
Embodiment 2
[0021] A quadruple active bacterial preparation for treating diarrhea, comprising 50% probiotic mixture, 10% xylooligosaccharide, and the balance being glucose;
[0022] The probiotic mixture includes freeze-dried powder of Bifidobacterium animalis, freeze-dried powder of Lactobacillus paracasei, freeze-dried powder of Enterococcus faecium and freeze-dried powder of Saccharomyces cerevisiae.
[0023] The live bacteria ratio of Bifidobacterium animalis, Lactobacillus paracasei, Enterococcus faecium and Saccharomyces cerevisiae is 5:5:1:1.
[0024] The number of live bacteria of Bifidobacterium animalis B1014 strain ≥ 2.0×10 9 CFU / g, live bacteria count of Lactobacillus paracasei L1056 strain ≥2.0×10 9 CFU / g, the number of live bacteria of Enterococcus faecium EF1012 strain ≥ 2.0×10 8 CFU / g, the number of live bacteria of Saccharomyces cerevisiae S1072 strain ≥2.0×10 8 CFU / g.
[0025] The survival rate of Lactobacillus paracasei, Enterococcus faecium and Saccharomyces cerevi...
Embodiment 3
[0028] A quadruple active bacterial preparation for treating diarrhea, comprising 25% probiotic mixture, 8% xylooligosaccharide, and the balance being glucose;
[0029] The probiotic mixture includes freeze-dried powder of Bifidobacterium animalis, freeze-dried powder of Lactobacillus paracasei, freeze-dried powder of Enterococcus faecium and freeze-dried powder of Saccharomyces cerevisiae.
[0030] The live bacteria ratio of Bifidobacterium animalis, Lactobacillus paracasei, Enterococcus faecium and Saccharomyces cerevisiae was 15:15:2:2.
[0031] The number of live bacteria of Bifidobacterium animalis B1014 strain ≥ 2.0×10 9 CFU / g, live bacteria count of Lactobacillus paracasei L1056 strain ≥2.0×10 9 CFU / g, the number of live bacteria of Enterococcus faecium EF1012 strain ≥ 2.0×10 8 CFU / g, the number of live bacteria of Saccharomyces cerevisiae S1072 strain ≥2.0×10 8 CFU / g.
[0032] The survival rate of Lactobacillus paracasei, Enterococcus faecium and Saccharomyces cere...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com