Heparin skeleton synthase and encoding gene and application thereof
A technology for coding genes and skeletons, which is applied to heparin skeleton synthase and its coding genes and application fields to achieve the effect of improving transferase activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
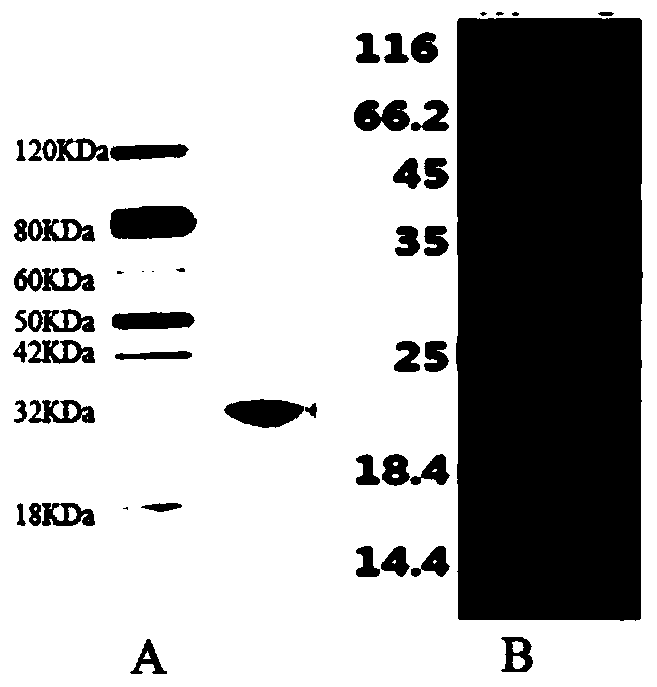
[0043] Example 1. Preparation of Heparin Skeleton Synthase GaGlcNAc-T
[0044] (1) Construction of expression strains
[0045] The recombinant vector pET28a-His-GaGlcNAc-T (synthesized by Nanjing GenScript Co., Ltd.) containing the heparin backbone synthase coding gene (SEQ ID NO.1) was transformed into Escherichia coli BL21 (DE3) competent cells to construct recombinant strains, Kanamycin (100 μg / mL) was cultured on the LB plate for 12h, and the transformants were screened (negative control experiment), and activated to obtain GaGlcNAc-T positive transformants; the amino acid sequence of the heparin backbone synthase was as SEQ ID NO.2 shown;
[0046] The recombinant vector pET28a-His-GaGlcNAc-T(N56D) (synthesized by Nanjing GenScript Co., Ltd.) containing the heparin backbone synthase mutant coding gene (SEQ ID NO.3) was transformed into Escherichia coli BL21(DE3) competent cells, Construct recombinant bacterial strain, and obtain GaGlcNAc-T (N56D) positive transformant ac...
Embodiment 2
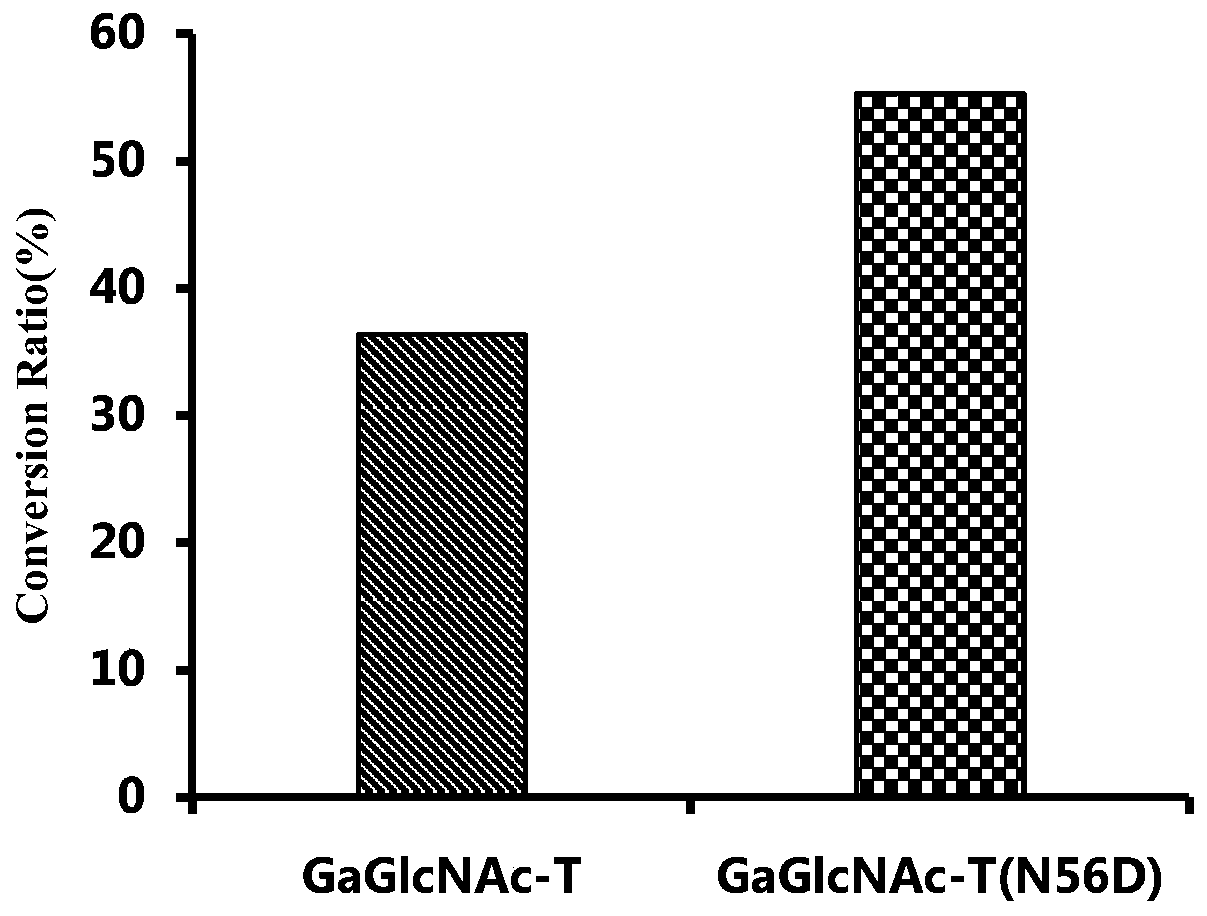
[0053] Example 2. Activity verification of heparin backbone synthase GaGlcNAc-T
[0054] (1) Verification of GlcNAc transferase activity of heparin backbone synthase GaGlcNAc-T
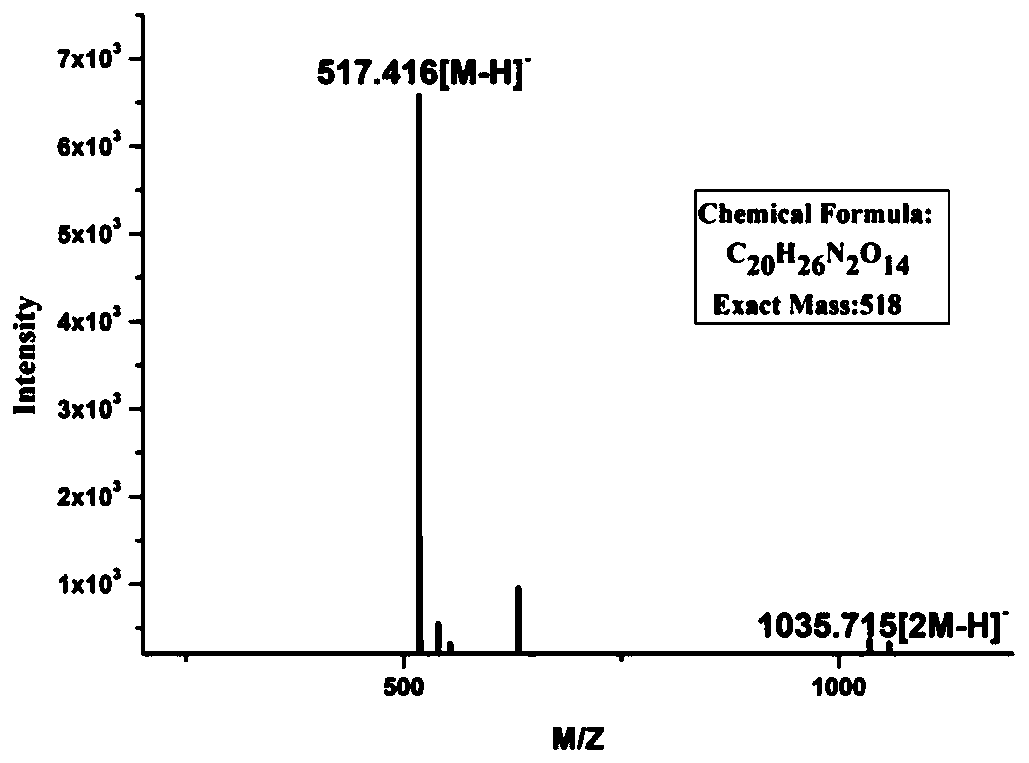
[0055] Using commercial GlcA-pNP (0.2mM final concentration) as the acceptor substrate, UDP-GlcNAc (0.3mM final concentration) as the donor substrate, the heparin backbone synthase GaGlcNAc-T prepared in Example 1 and The heparin backbone synthase mutant GaGlcNAc-T(N56D) was reacted as a protease, and the reaction system was shown in Table 2; the reaction was placed in a water bath at 37°C for 4 hours, and heated in boiling water for 5 minutes to inactivate the protease to terminate the reaction. After filtering with a 0.22 μm filter membrane, perform HPLC detection according to the method described in Table 1. The pNP group of the monosaccharide acceptor has specific absorption at the ultraviolet detection wavelength of 310 nm, and the flow rate of the mobile phase is 0.5 mL / min.
[0056] Table 2. R...
Embodiment 3
[0062] Example 3 Study on the Properties of Heparin Skeleton Synthase GaGlcNAc-T
[0063] (1) Determination of optimal reaction pH of enzyme in vitro reaction
[0064] The reaction system is shown in Table 2. The heparin backbone synthase GaGlcNAc-T prepared in Example 1 was used as the protease, and the pH of the buffer solution remained unchanged. The Tris-HCl buffer solution was replaced with Tris-HCl with different pH values. / PBS / MES buffer, respectively set 3.0, 4.0, 5.0, 6.0, 6.5, 7.0, 7.5, 8.0, 10, a total of 9 pH gradient points, and each gradient has three parallel groups. All the other processing conditions are with embodiment 2.
[0065] The result is as Figure 4 As shown, the results show that the heparin backbone synthase GaGlcNAc-T is active in a wide pH range (6.0-8.5), and the optimum pH of the reaction is 6.5.
[0066] (2) Determination of the optimal metal ion for the in vitro reaction of the enzyme
[0067] The reaction system is shown in Table 2. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com