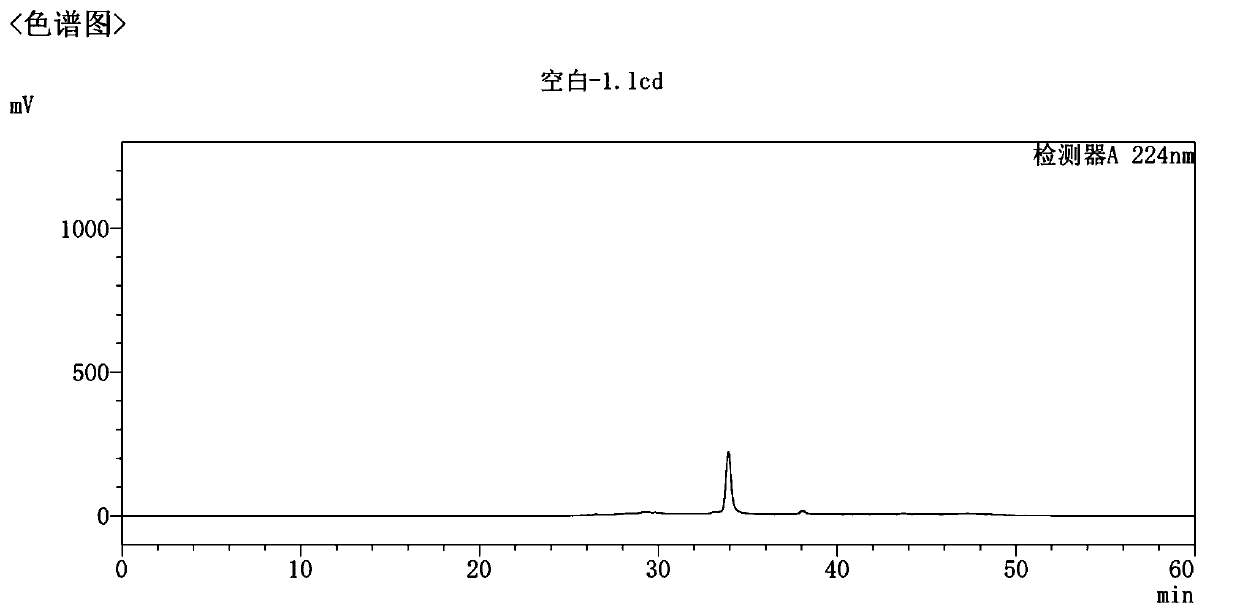
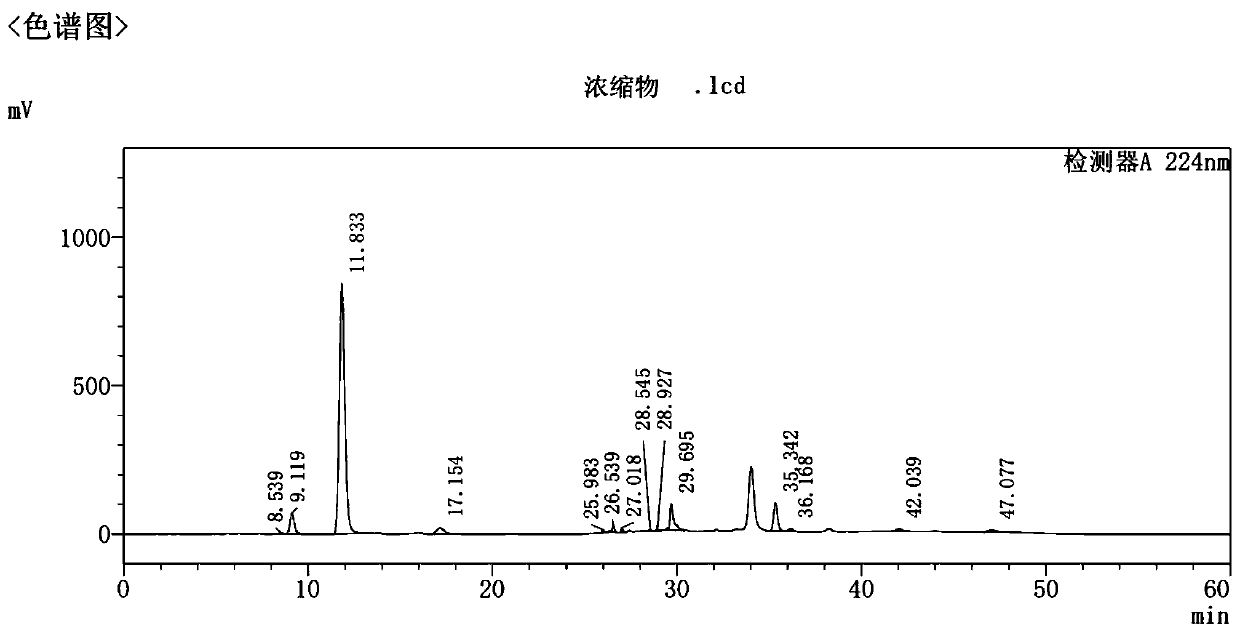
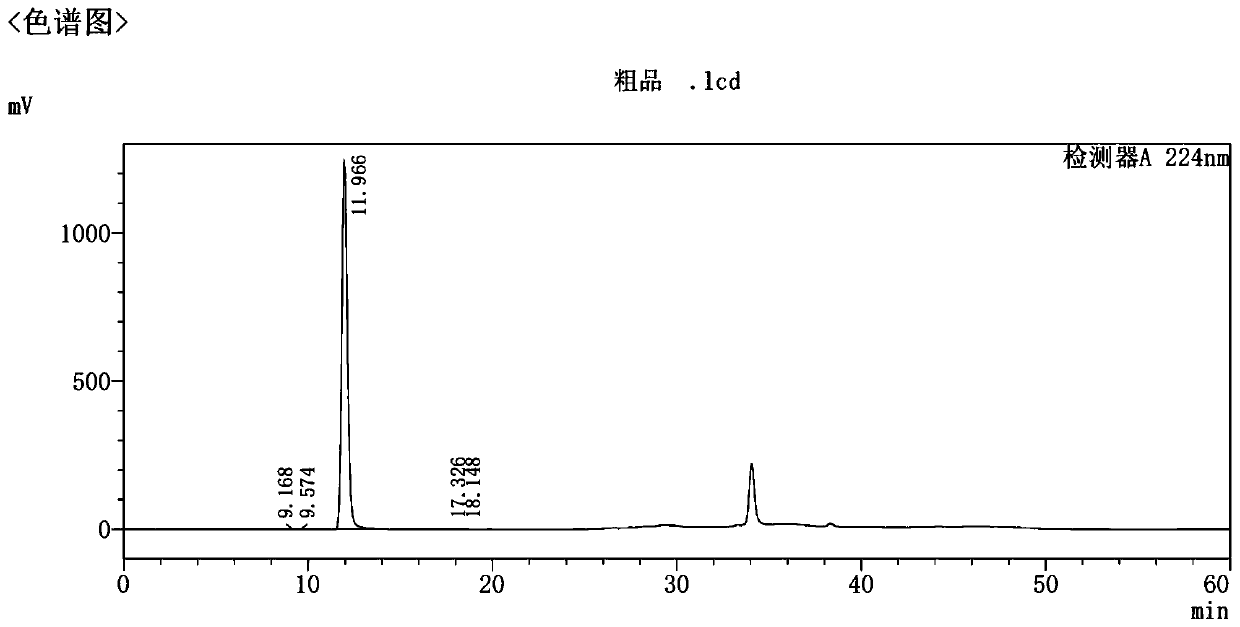
Preparation and refining method of high-purity empagliflozin
A purification method and technology for empagliflozin, which are applied in the field of preparation and purification of high-purity empagliflozin, can solve the problems of increasing production costs, harsh use conditions, and many times of purification and purification, saving production costs and being beneficial to The effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1: the preparation of crude product
[0069] Add SM1 (2000g) and THF (3575ml) to a dry 20L four-necked bottle under nitrogen protection, install a tail gas absorption device, stir and dissolve completely at 20-25°C, cool the inner temperature to -16°C, and add (1.3N) dropwise Isopropylmagnesium chloride-lithium chloride THF solution 4410ml, control the internal temperature at -14±2°C during the dropwise addition, drop it in 1.5 hours, continue to stir and react for 0.5 hours, add 2251.5g SM2 dropwise between the temperature control -14±2°C, After 50 minutes of dropwise addition, continue to keep warm for 1 hour, and TLC detects that the reaction is basically complete (developing solvent: petroleum ether / ethyl acetate=3 / 1).
[0070] Control the internal temperature below 5°C and add 6,000ml of 10% citric acid aqueous solution dropwise. After 20 minutes, the dropwise addition is completed, and continue to stir for 5 minutes. Separate the upper organic phase, ex...
Embodiment 2
[0076] Embodiment 2: the refinement of embodiment 1 crude product
[0077] Refining: Add 200 g of the crude product into a 2 L single-necked bottle, add 400 mL of ethanol, and 800 mL of (1N) NaOH aqueous solution. Heat to reflux to dissolve the clear. Add 10 g of activated carbon, stir for 1 hour, and filter with suction. Heat (ethanol: water = 1:2) to wash the filter cake. The filtrate was heated to reflux and added to 6.4 L of (1N) NaOH aqueous solution at 80°C. After the dropwise addition was completed, the mixture was stirred at 15-20° C. for 16 hours. Suction filtration, the solid was washed with purified water and suction filtration until neutral. The wet product was air-dried at 50° C. for 15 hours to obtain 192.5 g of dry product. Yield: 96.25%. HPLC: 99.97%. (See Figure 4 )
Embodiment 3
[0078] Example 3: Comparison of the solvent effects of the crystallization of the crude product of Empagliflozin
[0079] Using the oil of Example 1 as a raw material to study the solvent effect of crystallization of the crude product, the oil: impurity 1: 5.038%, impurity 2: 2.375%, and total other impurities: 16.242%.
[0080] In this example, the system numbered 6 is Example 1.
[0081] Solvent system No. 1-5: Add the mixed solvent or solvent in the stated proportion to the oil at room temperature at one time, then stir at 10-15°C for 15 hours, and filter with suction to obtain the crude product.
[0082] Table 1: Comparison of impurities and purity after crystallization of crude products in different solvents:
[0083]
[0084] As can be seen from Table 1, the present invention selects different crude product crystallization solvents, oily matter: dichloromethane: 1N NaOH aqueous solution is 1:3:3 in dosage ratio, and temperature crystallizes under the condition of 10 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com