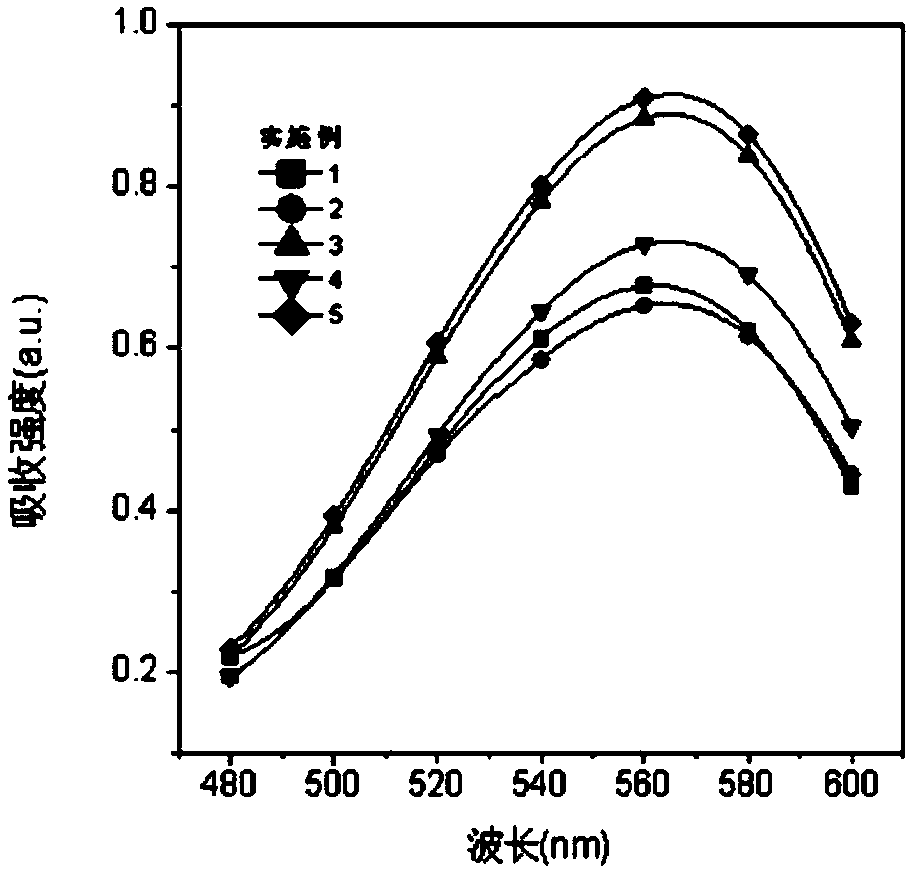
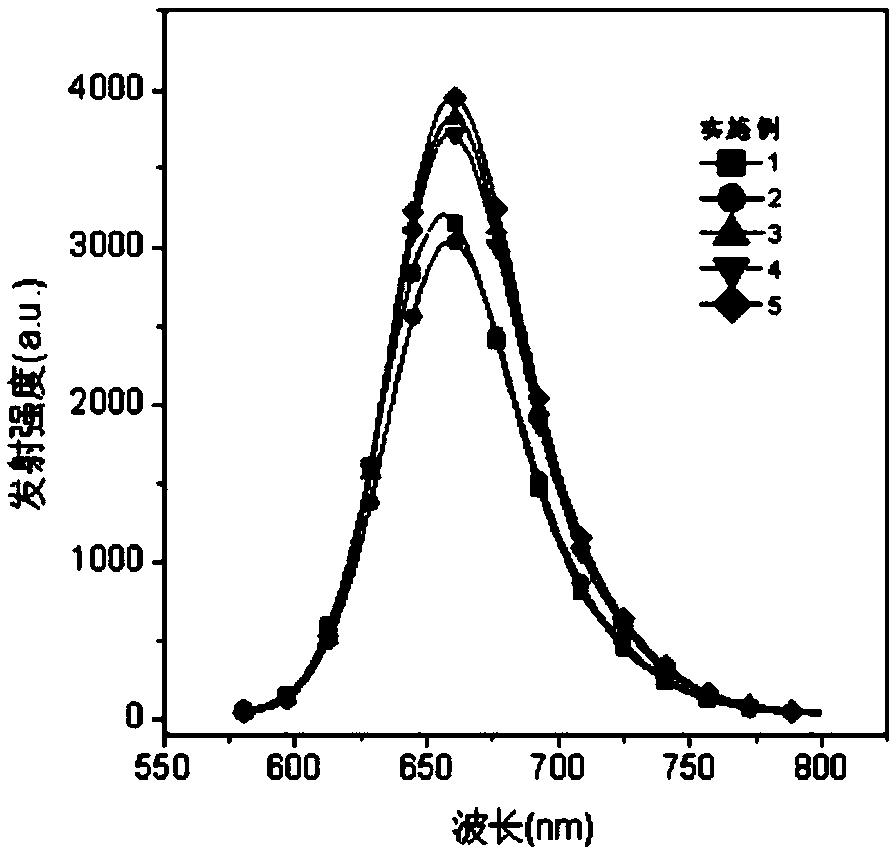
Near-infrared fluorescent dye based on coumarin skeleton and synthesis method thereof
A technology of coumarin skeleton and fluorescent dye, applied in the field of fluorescent dyes for biomedicine, can solve the problems of short life, high reactivity, low concentration, etc., and achieve the effect of avoiding the interference of self-fluorescence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Add 1.2mL (15.6mmol) N,N-dimethylformamide to 1.23mL (13.25mmol) phosphorus oxychloride, stir at room temperature for 30min, then add 5mL containing 339mg (1.56mmol) of formula I-1 4-(Diethylamino)coumarin in N,N-dimethylformamide solution, stirred and reacted at 60°C for 24 hours, after the reaction was completed, pour the reaction solution into ice water, wash with ethyl acetate and saturated saline The reaction solution was extracted, the organic phases were combined, and dry column chromatography (using a mixture of petroleum ether and ethyl acetate at a volume ratio of 30:1 as the eluent) gave the compound of formula II.
[0041]
[0042] 2. Dissolve 98mg (0.4mmol) of the compound of formula II and 155mg (1mmol) of 4-acetonitrile pyridine hydrochloride in 2mL of ethanol, add 221μL (1.6mmol) of triethylamine, stir and react at 40°C for 12 hours, and filter to obtain Compound of formula III.
[0043]
[0044] 3. Dissolve 104 mg (0.3 mmol) of the compound of...
Embodiment 2
[0048] Step 1 and Step 2 of this embodiment are the same as those of Embodiment 1. In step 3 of this example, 104 mg (0.3 mmol) of the compound of formula III was dissolved in 4 mL of N,N-dimethylformamide, 272 μL (3 mmol) of bromopropane was added, and the reaction was stirred at 80°C for 12 hours. Extract the reaction solution with dichloromethane and saturated brine, combine the organic phases, and dry column chromatography after spin-drying (using a mixture of dichloromethane and methanol with a volume ratio of 40:1 as the eluent) to obtain formula IV-2 The indicated fluorochromes were obtained in 75% yield.
[0049]
Embodiment 3
[0051] In this example, bromopropane in Example 2 was replaced with bromohexane in an equimolar amount, and the other steps were the same as in Example 2 to obtain the fluorescent dye shown in Formula IV-3 with a yield of 78%.
[0052]
[0053] The structural characterization data of the resulting product are: 1 H NMR (400MHz, DMSO) δ9.02(d, J=6.9Hz, 2H), 8.83(s, 1H), 8.37(d, J=16.6Hz, 1H), 8.31(d, J=6.9Hz, 2H ), 7.66(d, J=9.1Hz, 1H), 6.88(dd, J=9.1, 2.2Hz, 1H), 6.68(d, J=2.0Hz, 1H), 4.55(t, J=7.4Hz, 2H ), 3.55(q, J=6.9Hz, 4H), 1.89(d, J=2.5Hz, 2H), 1.28(s, 5H), 1.16(t, J=7.0Hz, 5H), 0.85(t, J =6.6Hz,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com