Electrochemical polymerization preparation of triphenylamine derivative conjugated polymer material and applications
An electrochemical and polymer thin film technology, applied in the fields of organic chemistry, color-changing fluorescent materials, chemical instruments and methods, etc., can solve the problems of limiting the application of electrochromic field, difficult to prepare by electrochemical polymerization, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1: Synthesis of 2,3,8,9,14,15-six (4-dianilinophenyl)-hexaazyridine:
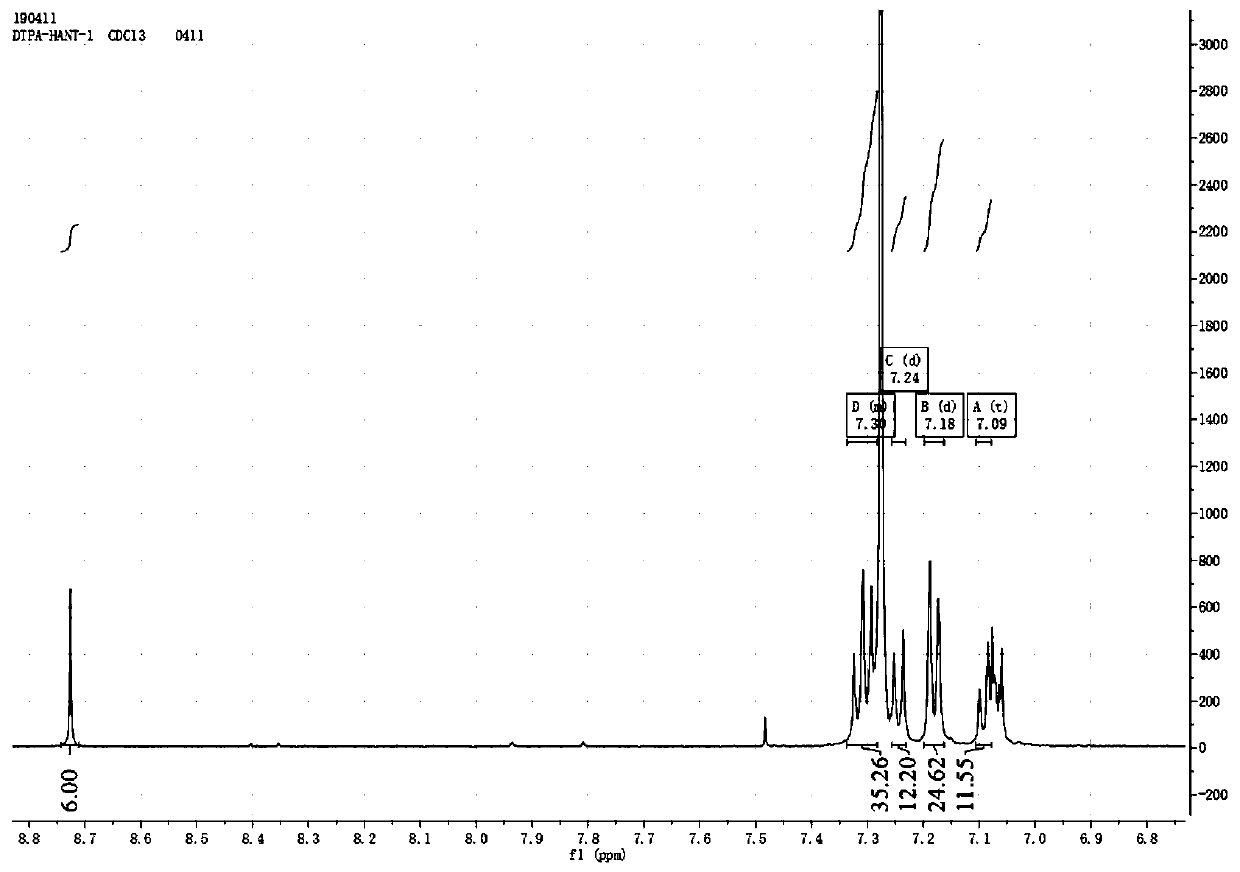
[0065] Add hexahydrate and cyclohexanone (60mg), 4,5-bis(N,N-diphenylbenzene)-1,2-diaminobenzene (600mg) into a pre-washed and dried 50ml two-necked flask, nitrogen protection Then add glacial acetic acid (20ml), heat up to 120°C, and react for 15h. After the reaction was completed, it was washed and extracted three times with dichloromethane and deionized water, and the organic phase was collected, dried over anhydrous magnesium sulfate, concentrated under reduced pressure, and separated and purified by column chromatography. Using 200-300 mesh silica gel as the stationary phase, eluting with a dichloromethane / ethyl acetate mixture with a volume ratio of 2:1 as the mobile phase, collecting the eluate containing the target compound, distilling off the solvent under reduced pressure and drying to obtain Red solid 344mg, yield 85%. 1 H NMR (500MHz, CDCl3) δ8.73 (s, 6H), 7.34-7.28 (m,, 36H)...
Embodiment 2
[0066] Example 2: Synthesis of 4,5-bis(N,N-diphenylbenzene)-1,2-diaminobenzene:
[0067] Add 4-boric acid triphenylamine (773mg), 4,5-dibromo-o-diaminobenzene (138mg), Pd(PPh 3 ) 2 Cl 2 (40mg), K 2 CO 3 (424mg), after nitrogen protection, 30ml of toluene and 2ml of deionized water were added, the temperature was raised to 80°C, and the reaction was carried out for 24h. After the reaction, the mixture was washed and extracted with dichloromethane and water several times, and the organic phase was collected, dried over anhydrous magnesium sulfate, concentrated under reduced pressure, and separated and purified by column chromatography. Use 200-300 mesh silica gel as the stationary phase, and use dichloromethane / petroleum ether mixed solution with a volume ratio of 3:1 as the mobile phase for elution, collect the eluate containing the target compound, distill off the solvent under reduced pressure and dry it, then TLC was used to obtain 488 mg of pink solid with a yield of 8...
Embodiment 3
[0068] Example 3: Preparation and characterization of 2,3,8,9,14,15-hexa(4-dianilinophenyl)-hexaazyridine polymer film:
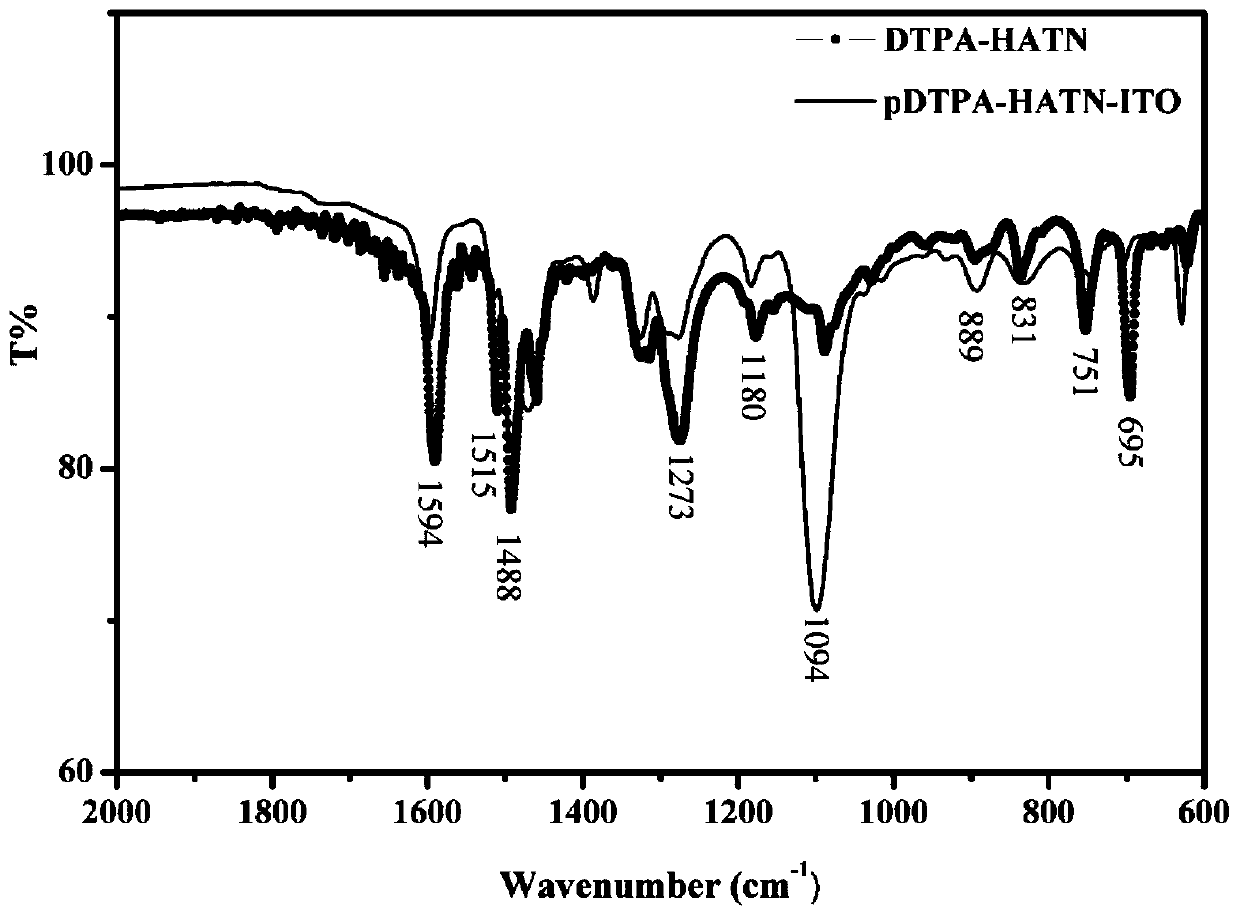
[0069] Characterization of 2,3,8,9,14,15-hexa(4-dianilinophenyl)-hexaazyridine monomer in polymer film by infrared spectroscopy (see attached image 3 ), it can be seen from the figure that the compound monomer and the polymer film have the characteristic infrared absorption peaks of the triphenylamine unit and the central core hexaazyridine at the same time. Located at 1594, 1515, 1452cm -1 The absorption peak is the skeleton vibration of substituted benzene, 1488cm -1 and 1360cm -1 The absorption peaks at are respectively the C=N stretching vibration and the C-N stretching vibration in the naphthalene heterocycle, 1273 and 1180cm -1 The absorption peak at corresponds to the C-N stretching vibration in triphenylamine and the C-H in-plane bending vibration of substituted benzene, 889 and 831 cm -1 Corresponding to 1,2,4 trisubstituted benzene C-H out-of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com