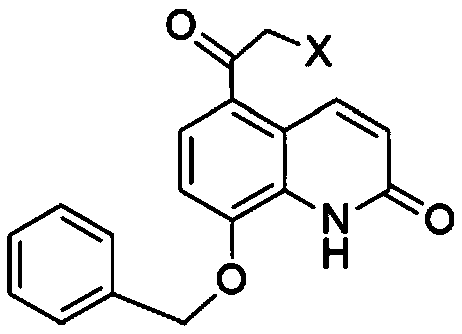
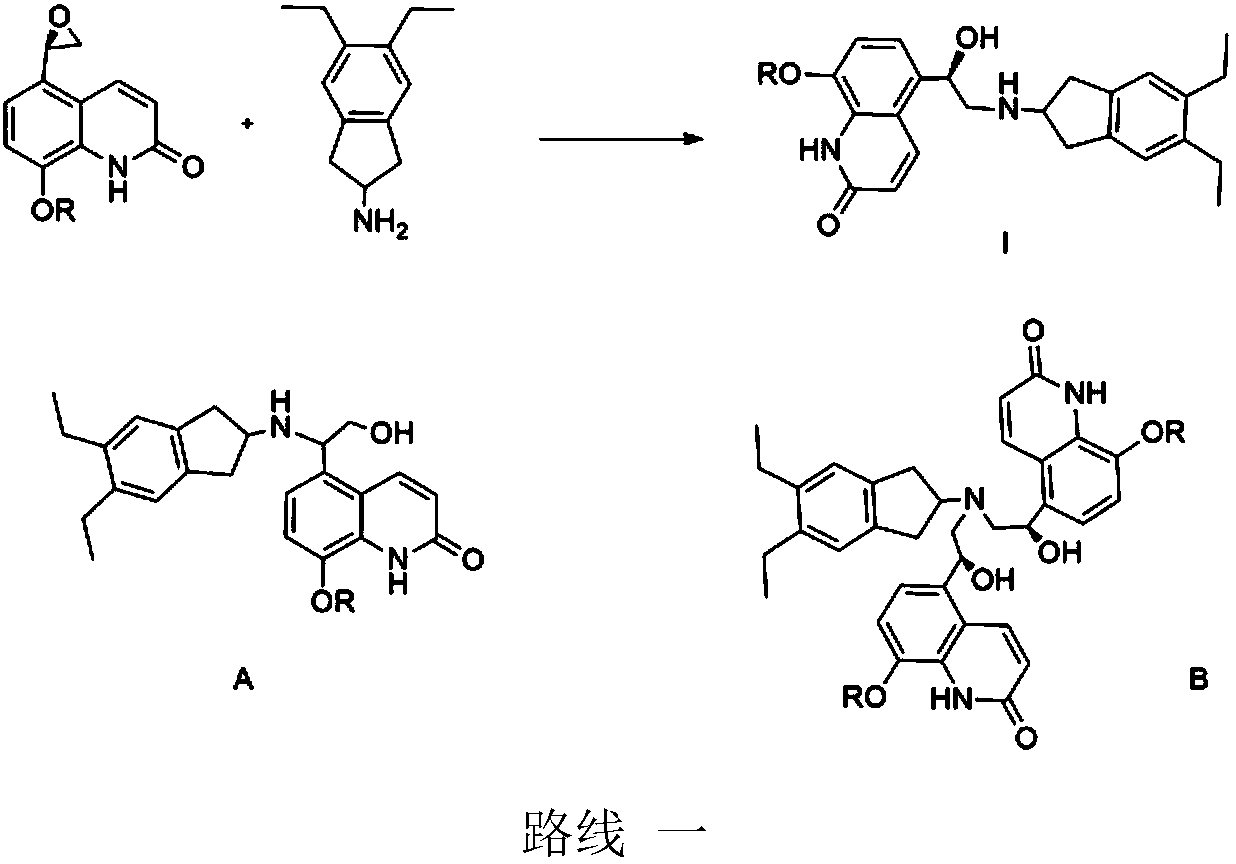
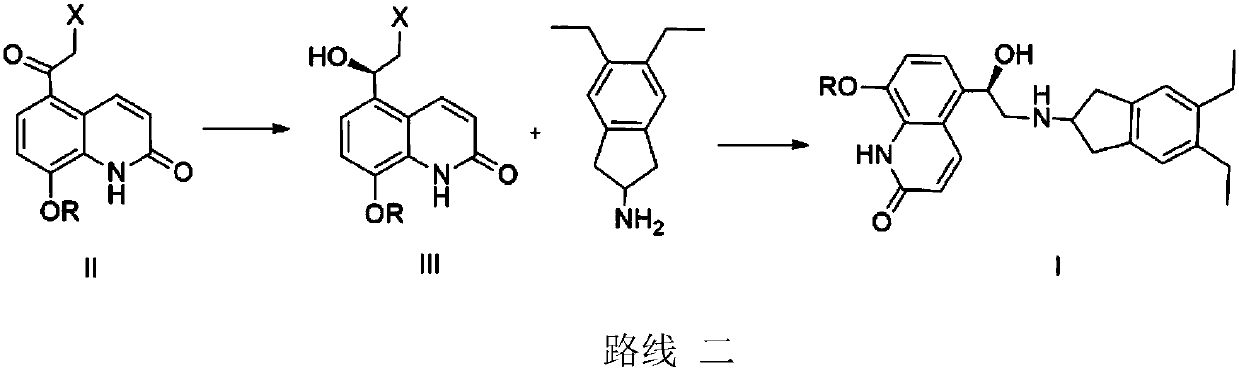
Application of carbonyl reductase and carbonyl reductase mutants in Indacaterol drug intermediates
A carbonyl reductase and enzymatic activity technology, which is applied in the field of bioengineering, can solve the problems of large steric hindrance of substrate II and no conversion activity of carbonyl reductase, and achieves the effects of improved conversion activity, simple and convenient preparation method, and environmental friendliness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: RasADH and mutant gene obtained
[0019] 1.1 Synthesis of RasADH gene and acquisition of its mutant gene
[0020] The RasADH gene is derived from Ralstonia sp.DSM 6428, and has a crystal structure (PDB: 4BMS). According to the codon preference of Escherichia coli, the gene is optimized and sent to the company for synthesis. The codon-optimized genes are respectively A NdeI (CATATG) restriction site was added at the 5' end, and an XhoI (CTCGAG) restriction site was added at the 3' end, and cloned into the PET21a vector. Since the crystal structure of RasADH is known, Discovery Studio 4.1 software was used to dock the substrate II with its three-dimensional structure to obtain the interaction relationship between the substrate and the protein. According to the docking results, the amino acids 189, 191, 202, and 205 near the active center were selected for site-directed mutagenesis, and the PET21a plasmid with the RasADH gene was used as a template and prime...
Embodiment 2
[0035] Embodiment 2: the construction of RasADH and its mutant expression bacteria
[0036] After the digested and purified PCR product was transformed into Escherichia coli BL21(DE3) competent cells by chemical transformation, single clones were picked and placed in 4 mL of LB medium containing ampicillin (10 mg / mL), and fresh bacterial liquid was taken Sent to a sequencing company for sequencing, and the sequencing results showed that the correct mutant was the strain expressing the RasADH mutant.
Embodiment 3
[0037] Example 3: Induced expression of RasADH and mutants thereof
[0038] Prepare 50 mL of seed liquid, and the medium is LB liquid medium (peptone 10 g / L, yeast powder 5 g / L, NaCl 10 g / L), pick a single colony of genetically engineered bacteria with an inoculation loop and insert it into the medium, at 37 °C, Incubate overnight at 200 rpm. The seed solution cultivated overnight was transferred to the fermentation medium with an inoculum size of 1%, and cultivated at 25°C and 200rpm for 20h. After 5 mL of the fermentation broth was concentrated and ultrasonicated, the activity of RasADH and its mutants was detected. Enzyme activity definition of RasADH and its mutants: the amount of enzyme needed to consume 1 μmol NADPH per minute is 1 enzyme activity unit (U).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com