Preparation method of Anhydroicaritin oral micelle preparation
A technology of icariin and micelles, which is applied in anti-inflammatory agents, pharmaceutical formulas, non-central analgesics, etc., can solve the problem of poor dissolution of icariin, difficulties in meeting the requirements of oral micelles, biological Problems such as low utilization can achieve the effects of improving drug safety, eliminating conventional hydration and freeze-drying processes, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
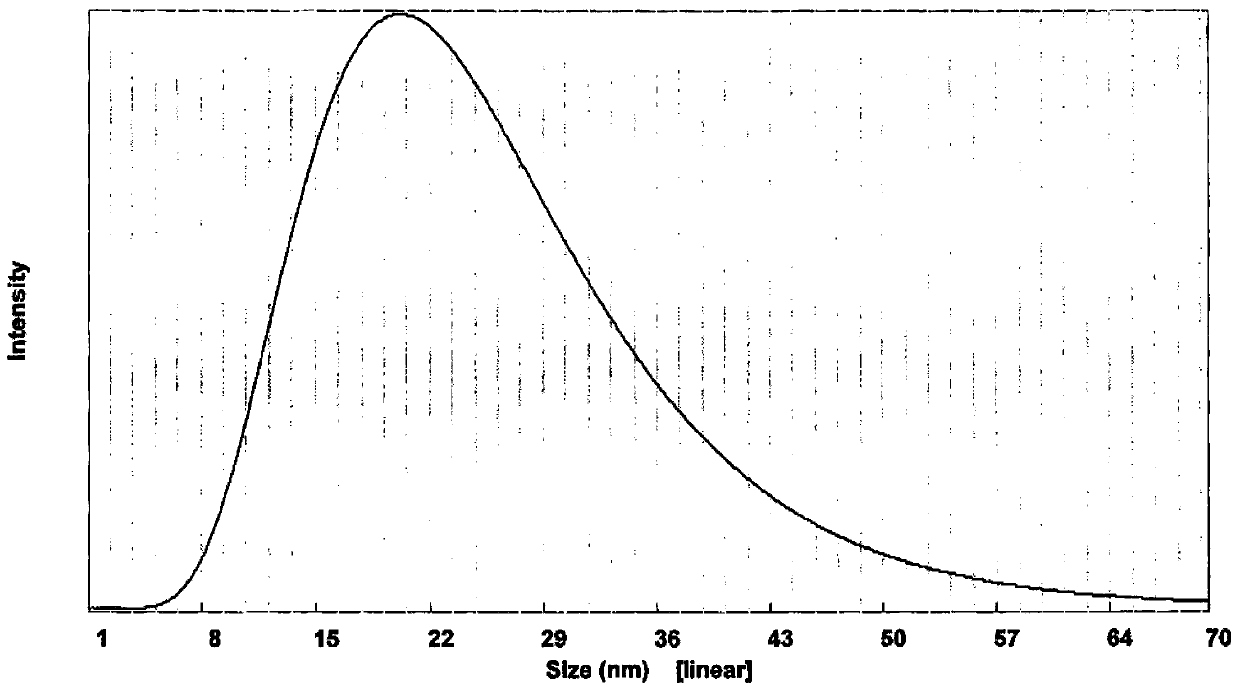
Image
Examples
Embodiment 1
[0033] 1) Prescription
[0034] 1 part icariin
[0035] PEG 3000 -PLA 3000 5 copies
[0036] Lactose 60 parts
[0037] 2) Preparation steps:
[0038] a) Weighing the prescription amount of icariin and mPEG-PLA, adding 12 parts of acetonitrile, stirring to dissolve;
[0039] b) adding the prescription amount of lactose to the mixed solution in step a), and preparing the soft material through a wet mixing granulator;
[0040] c) Make wet granules through a 18-mesh sieve, transfer the wet granules into a fluidized granulator, and dry at 40°C;
[0041] d) The dried granules are sieved through a 12-mesh sieve, and packed separately.
Embodiment 2
[0043] 1) Prescription
[0044] 1 part icariin
[0045] PEG 2400 -PLA 5600 2 copies
[0046] 50 parts of sucrose
[0047] 2) Preparation steps:
[0048] a) Weigh the prescription amount of icariin and mPEG-PLA, add 10 parts of tetrahydrofuran, and stir to dissolve it;
[0049] b) adding the prescription amount of sucrose to the mixed solution of step a), and preparing the soft material through a wet mixing granulator;
[0050] c) Make wet granules through a 18-mesh sieve, transfer the wet granules into a fluidized granulator, and dry at 40°C;
[0051] d) The dried granules are sieved through a 12-mesh sieve, and packed separately.
Embodiment 3
[0053] 1) Prescription
[0054] Arctigenin 1 part
[0055] PEG 7000 -PLA 3000 10 copies
[0056] Dextrin 70 parts
[0057] 2) Preparation steps:
[0058] a) Weigh arctigenin and mPEG-PLA of the prescribed amount, add 14 parts of acetone, and stir to dissolve it;
[0059] b) Add the prescription amount of dextrin to the mixed solution in step a), and prepare the soft material through a wet mixing granulator;
[0060] c) Make wet granules through a 18-mesh sieve, transfer the wet granules into a fluidized granulator, and dry at 40°C;
[0061] d) The dried granules are sieved through a 12-mesh sieve, and packed separately.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com