Method for applying L-selenomethionine to animal cell oxidation resistance and product thereof
A technology of selenomethionine and animal cells, which is applied in the field of L-selenomethionine in the anti-oxidation of animal cells, can solve the problem of reduced glutathione peroxidase activity, reduced glutathione content, and increased ROS levels. High-level problems, to achieve the effect of improving the ability to resist oxidative damage, requiring extensive additions, and mitigating parameter changes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0076] The primary broiler hepatocytes in the embodiment are prepared by the following method:
[0077]Purchase commercially available 35-day-old healthy white-feathered AA broiler chickens, inject 3 mL of 200 U heparin sodium into the subwing vein after anesthesia, wash the whole body with fresh cleansing solution, and keep the broilers on the operating table. Open the abdominal cavity to expose the liver; ligate the perihepatic blood vessels, cut off all the ligated blood vessels, separate the connective tissue, remove the whole liver, wash the blood clot on the liver surface with 37°C D-Hank's buffer (no glucose), and Place in 37°C penicillin-streptomycin normal saline, and then perform perfusion. Find the hepatic portal vein on the ventral side of the chicken liver, cut a small opening, insert the catheter that removes the scalp needle into the vein, and ligate it. The hot perfusion solution A is slowly poured into the liver. When the liver expands slowly, use a needle to...
Embodiment 1
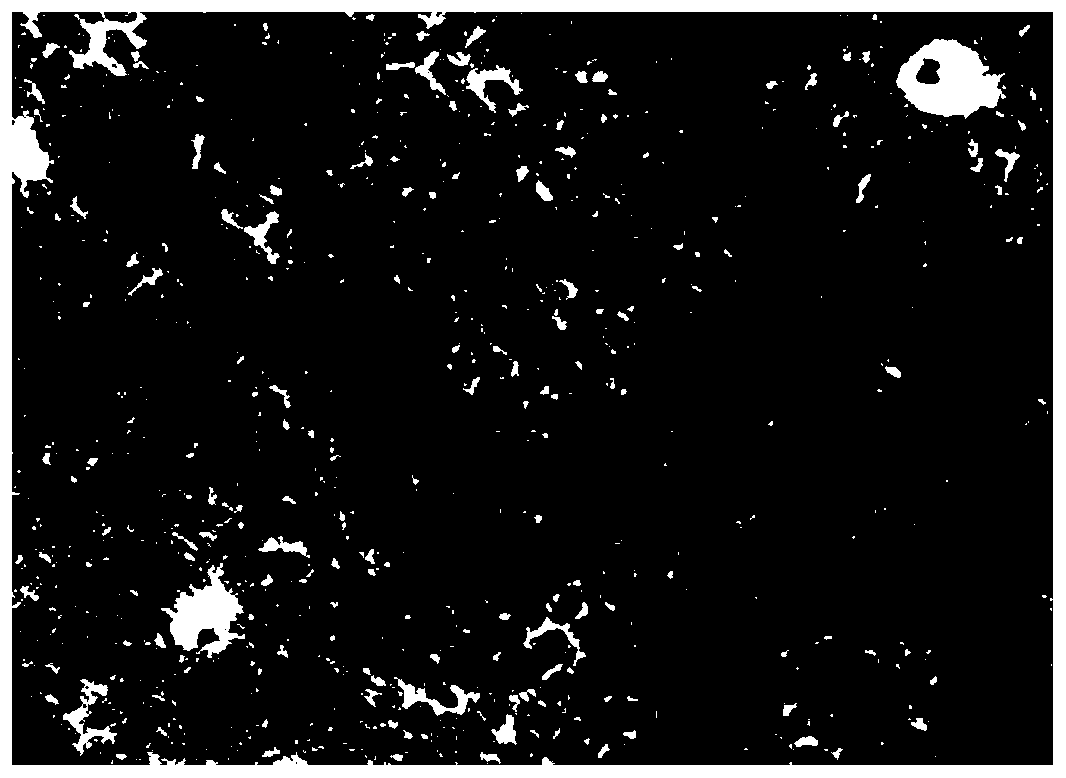
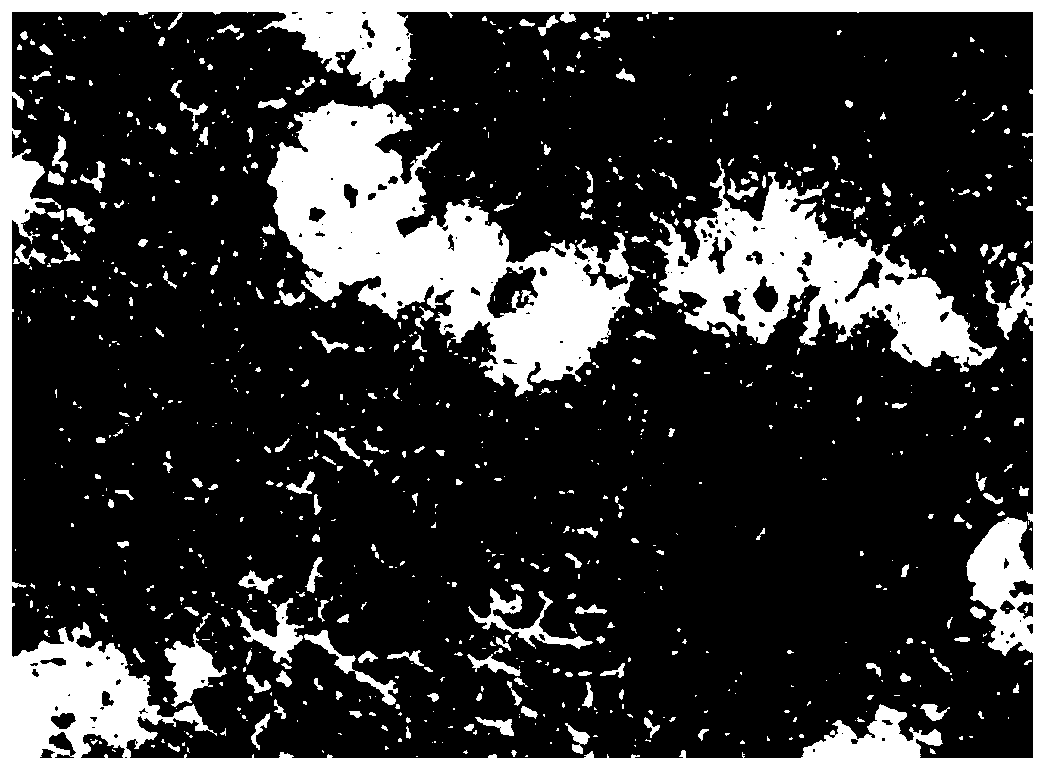
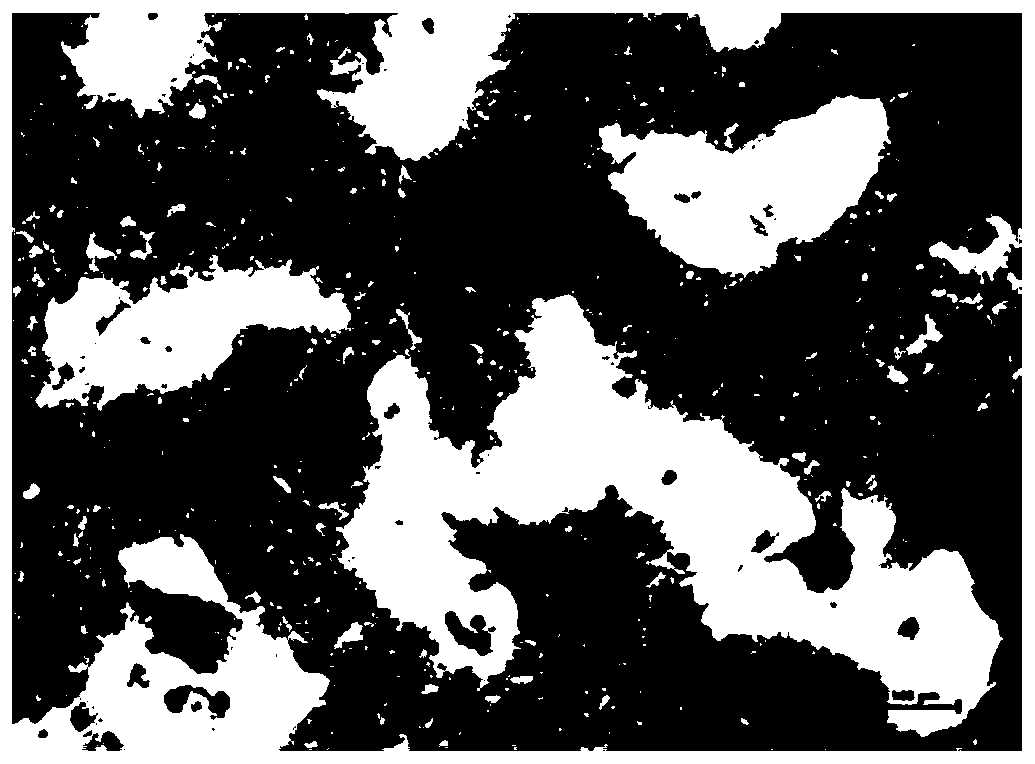
[0079] After the broiler hepatocyte suspension was cultured for 24 hours, the cells adhered to the wall, and the obtained broiler primary hepatocyte culture system was used as the animal cell culture system. (Note: 6-well plates and 96-well plates were plated with broiler liver cell suspension; the 6-well plates were subsequently used for HE staining to observe cell morphology, determination of malondialdehyde content, determination of glutathione content, glutathione peroxidation Determination of enzyme content; 96-well plate was used for cell activity determination; both were cultured for 24 hours as the primary broiler hepatocyte culture system in subsequent experiments.)
[0080] The culture system of broiler primary hepatocytes was not treated with T-2 toxin (that is, treated with 0nM T-2 toxin oxidative stress), cultured for 12h, and then added to the culture system of broiler hepatocytes at a concentration of 12μM L-seleno For the methionine working solution, adjust the...
Embodiment 2
[0082] The difference between Example 2 and Example 1 lies in the oxidative stress treatment: the culture system of broiler primary hepatocytes was treated with 10 nM T-2 toxin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com