A kind of imidazole substituted spirofluorene compound and application thereof
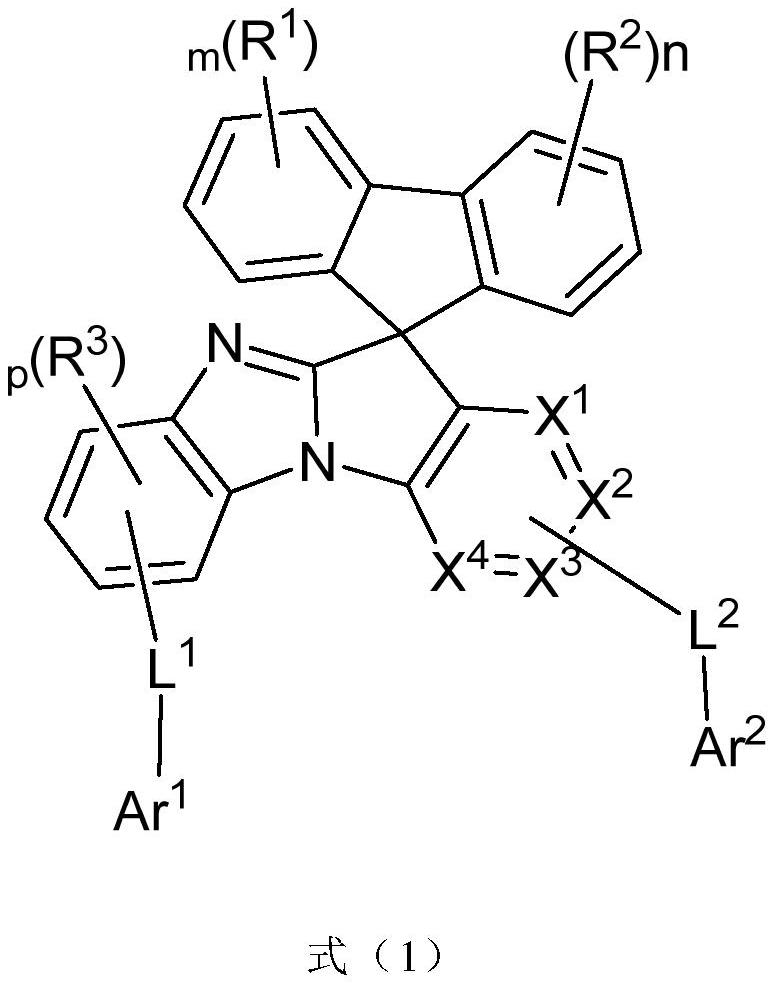
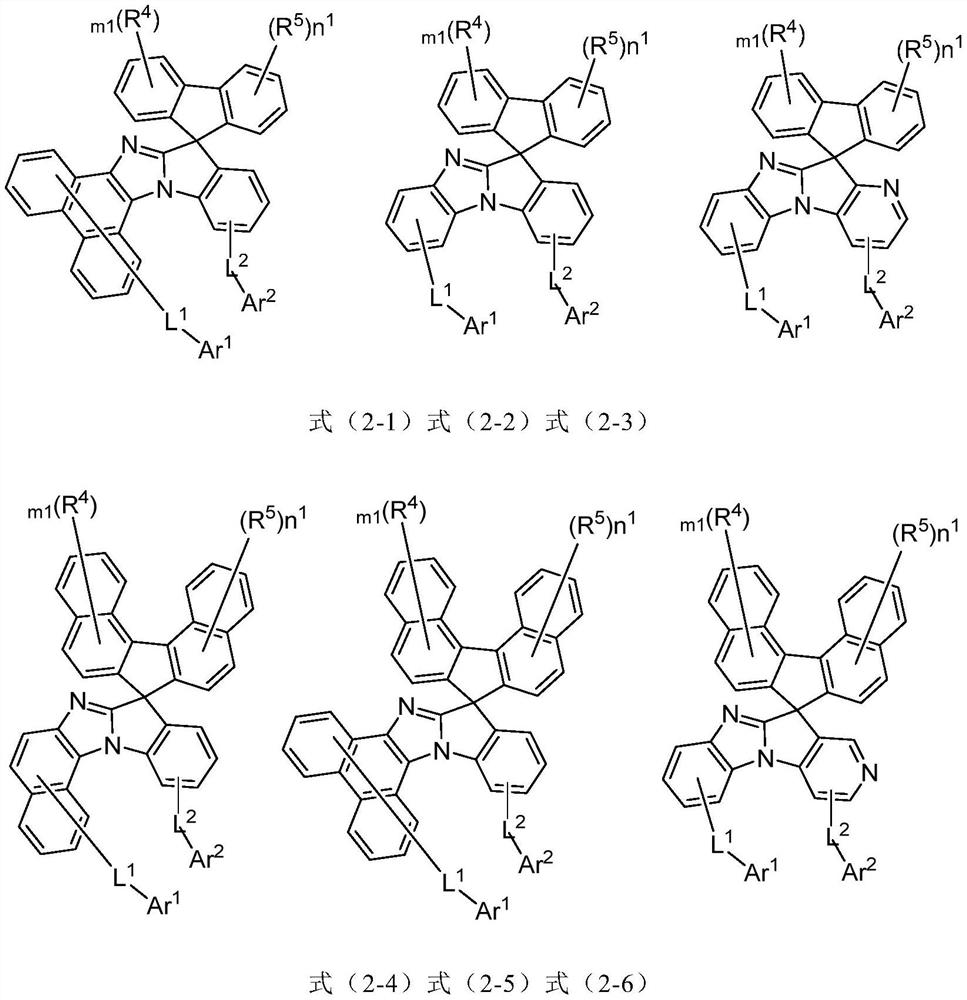
A general formula compound, unsubstituted technology, applied in the field of organic electroluminescent devices, imidazole substituted spirofluorene organic compounds, can solve the problems of weak electron transport ability, unbalanced carrier transport, etc., to achieve lower injection energy barrier, Effect of reducing efficiency roll-off and reducing turn-on voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0060] Synthesis Example 1: Synthesis of M1
[0061]
[0062] Synthesis of intermediate M1-1:
[0063] Add 11.8g (100mmol) of benzimidazole and 20.2g of 2-fluoro-4-bromobenzaldehyde (100mmol) into a freshly dried 500mL two-necked bottle, and add 20.7g (150mmol) of anhydrous potassium carbonate under nitrogen protection and 200 mL of dimethylsulfoxide (DMSO). The temperature was raised to 120°C and the reaction was continued for 20h. After the reaction was completed, the temperature was lowered to room temperature, and the reaction system was poured into 1 L of ice water stirred. At this time, a large amount of light yellow precipitates were produced. Suction filtration under reduced pressure and repeated washing with distilled water. The crude product was dissolved in dichloromethane, and the eluent was ethyl acetate:dichloromethane=1:10. Column chromatography gave off-white solid, 12.5 g, yield 42%. The molecular ion mass determined by mass spectrometry is: 298.02 (cal...
Synthetic example 2
[0068] Synthesis Example 2: Synthesis of M2:
[0069] According to the synthesis of M1, the steps were the same, and 5-bromo-2-fluorobenzaldehyde was used instead of 2-fluoro-4-bromo-benzaldehyde to obtain 7.2 g of white solid, with a yield of 69%. The molecular ion mass determined by mass spectrometry is: 521.16 (calculated value: 521.19); theoretical element content (%) C 38 h 23 N 3 : C, 87.50; H, 4.44; N, 8.06. Measured element content (%): C, 87.47; H, 4.42; N, 8.06. The above analysis results indicated that the obtained product was the expected product.
Synthetic example 3
[0070] Synthesis Example 3: Synthesis of M12:
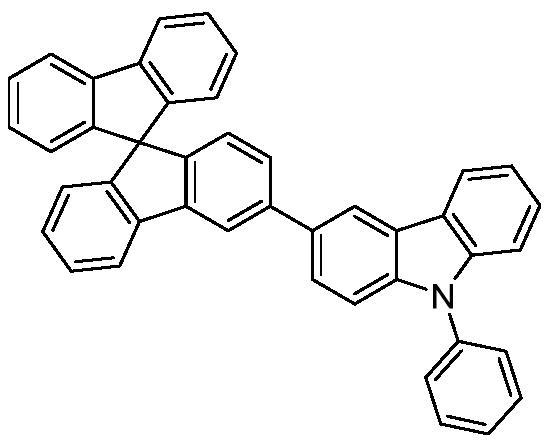
[0071] Take a dry 500mL two-necked bottle, add 8.68g (20mmol) M1-2, 7.2g (25mmol) 3-boric acid-9-phenylcarbazole, 4.14g (30mmol) anhydrous potassium carbonate, 230mg (0.2mmol) ) tetrakistriphenylphosphine palladium. After nitrogen replacement three times, 15 mL of distilled water and 250 mL of 1,4-dioxane were added, and heated to reflux for 12 h. After the solvent in the reaction system was distilled under reduced pressure, it was extracted with dichloromethane and washed with a large amount of water. After combining the organic phases, they were concentrated and subjected to column chromatography using dichloromethane: petroleum ether = 1:1 as the eluent to obtain a white solid, 10.3 g, with a yield of 86%. The molecular ion mass determined by mass spectrometry is: 597.20 (calculated value: 597.22); theoretical element content (%) C 44 h 27 N 3 : C, 88.42; H, 4.55; N, 7.03. Measured element content (%): C, 88.46; H, 4.54;...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com