Parathyroid hormone 1-34 nasal spray as well as preparation method and application thereof
A parathyroid hormone and nasal spray technology, applied in the field of biomedicine, can solve pain and other problems, achieve the effects of easy portability, prevention and treatment of osteoporosis, and improvement of compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0062] Preparation Example 1: Preparation of PTH(1-34) nasal spray sample 1
[0063] Prepare 0.4M pH7.4 phosphate buffer: 7.48 mg of sodium dihydrogen phosphate and 73.12 mg of disodium hydrogen phosphate are dissolved in water to prepare 0.4M phosphate buffer.
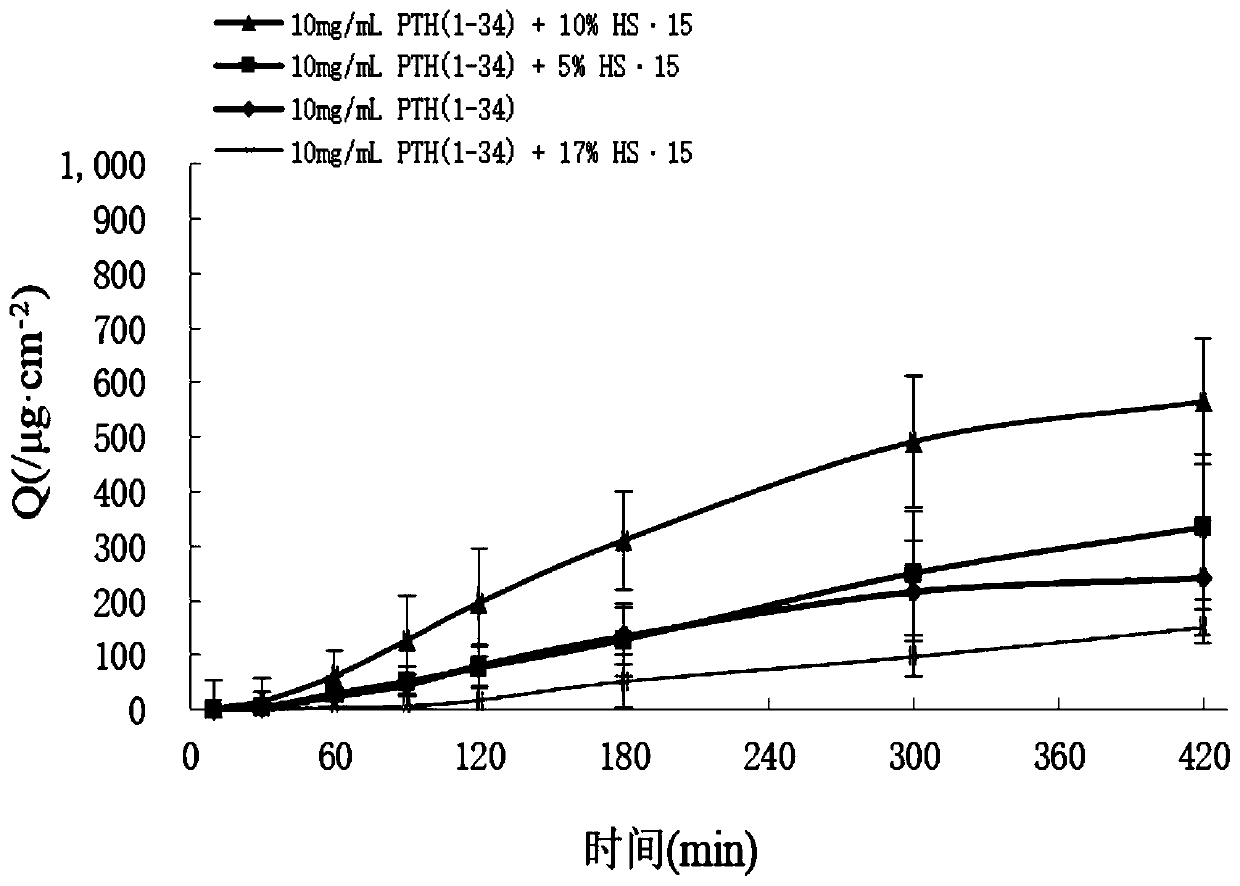
[0064] Weigh 80 mg of PTH (1-34) and dissolve it in water to prepare a 40 mg / mL mother solution, accurately measure 0.25 mL of PTH (1-34) mother solution, add an appropriate amount of 0.4M phosphate buffer (final concentration is 0.1M), add HS·15 100mg, then add 0.08mg of benzalkonium chloride, finally use water to make up to 1mL, mix thoroughly, put into a bottle and fill with nitrogen. The concentration of PTH(1-34) in the formulation was 10 mg / mL.
preparation example 2
[0065] Preparation Example 2: Preparation of PTH(1-34) nasal spray sample 2
[0066] Prepare 0.4M pH7.4 phosphate buffer: 7.48 mg of sodium dihydrogen phosphate and 73.12 mg of disodium hydrogen phosphate are dissolved in water to prepare 0.4M phosphate buffer.
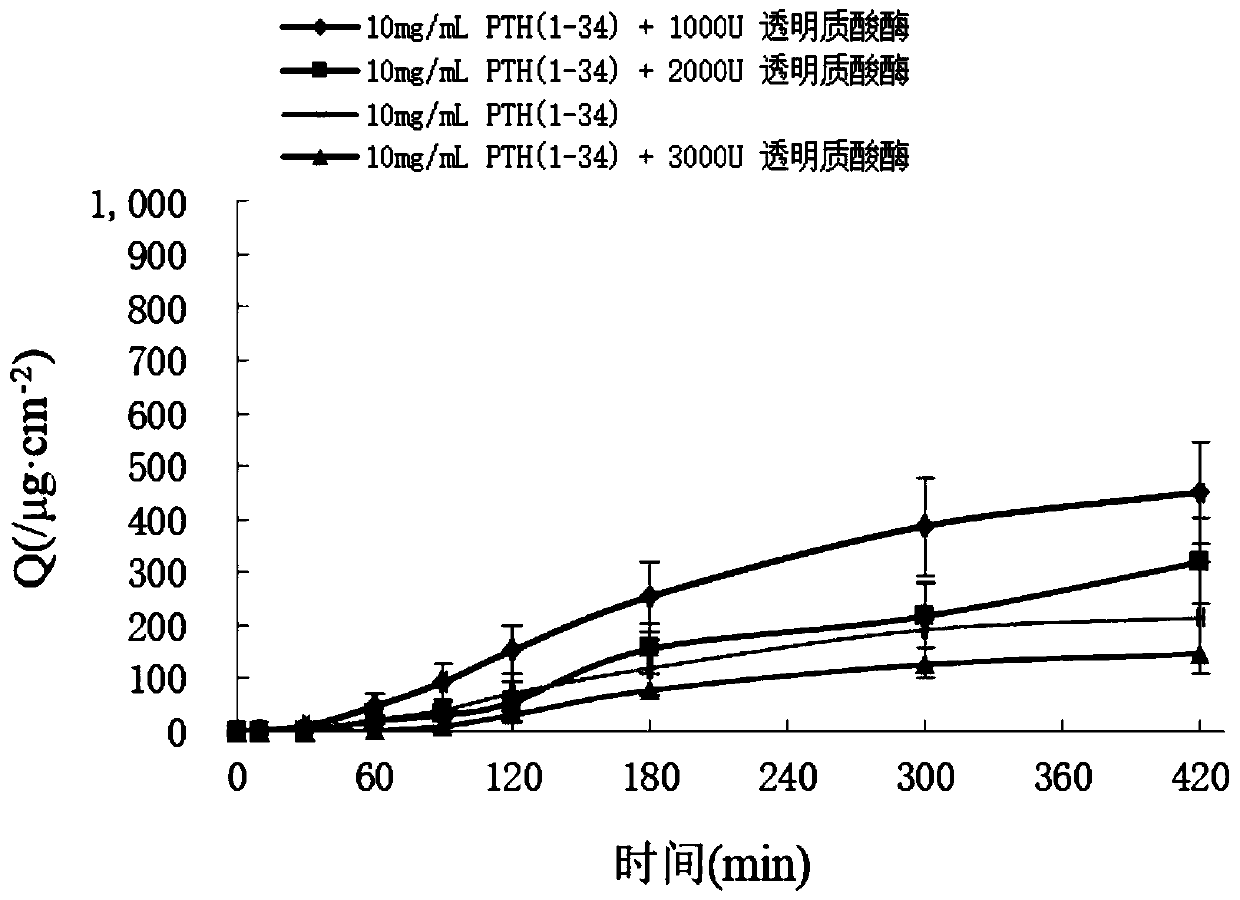
[0067] Weigh 80mg of PTH(1-34) and dissolve it in water to make 40mg / mL mother liquor, accurately measure 0.25mL of PTH(1-34) mother liquor, add appropriate amount of 0.4M pH7.4 phosphate buffer (final concentration is 0.1M), Add an appropriate amount of hyaluronidase 1000U, then add 0.08 mg of benzalkonium chloride, and finally dilute to 1 mL with water, mix well, put it into a bottle and fill it with nitrogen. The concentration of PTH(1-34) in the formulation was 10 mg / mL.
preparation example 3
[0068] Preparation Example 3: Preparation of PTH(1-34) nasal spray sample 3
[0069] First prepare 0.1M pH7.4 phosphate buffer: 1.87 mg of sodium dihydrogen phosphate and 18.28 mg of disodium hydrogen phosphate.
[0070] Next, prepare 0.4M pH7.4 phosphate buffer: 7.48 mg of sodium dihydrogen phosphate and 73.12 mg of disodium hydrogen phosphate are dissolved in water to prepare 0.4M phosphate buffer.
[0071] Weigh an appropriate amount HS·15, using distilled water to prepare a 400mg / mL concentrated solution.
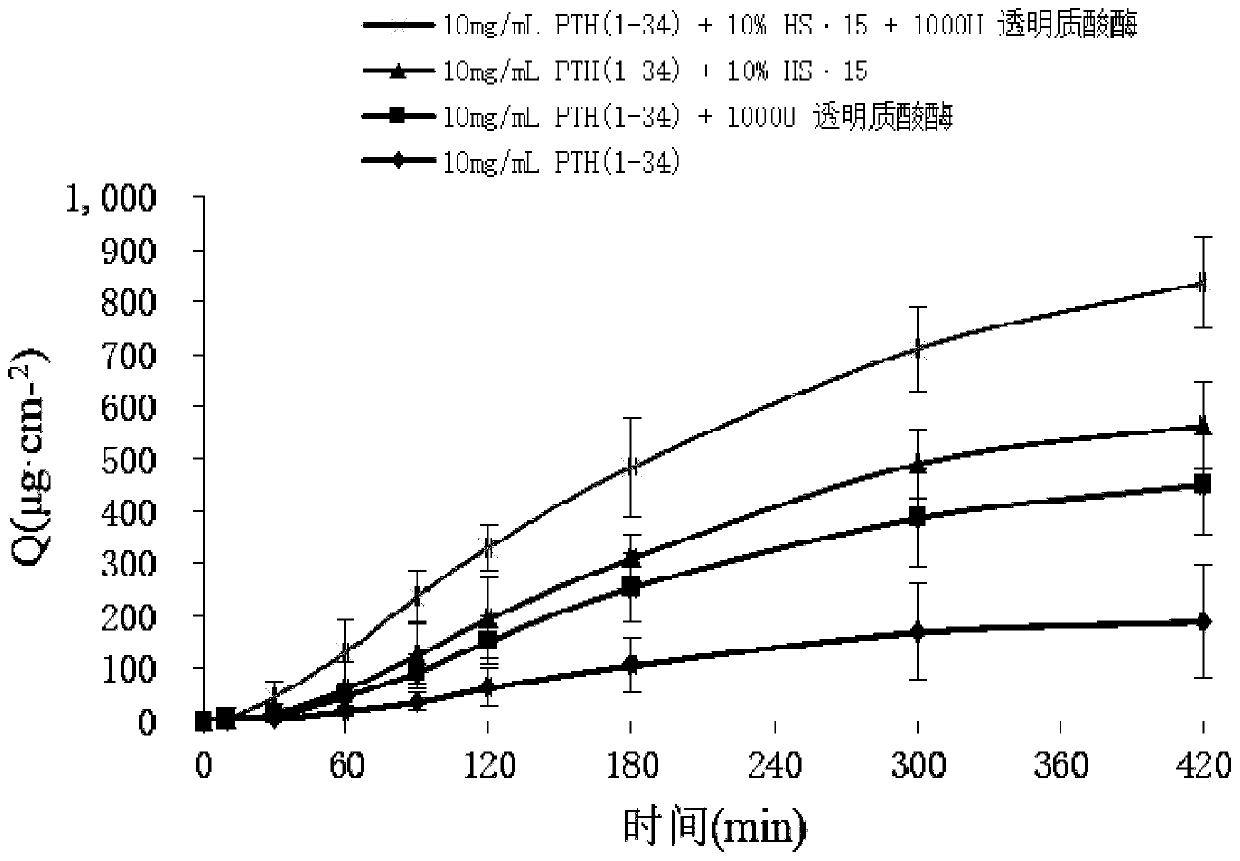
[0072] Weigh 80 mg of PTH (1-34) and dissolve it in water to prepare a 40 mg / mL mother solution, accurately measure 0.25 mL of PTH (1-34) mother solution, add an appropriate amount of 0.4M phosphate buffer (final concentration is 0.1M), add 0.25 mL 400mg / mL HS·15 concentrated solution, 1000 U of hyaluronidase, and 0.08 mg of benzalkonium chloride were added, and finally the volume was adjusted to 1 mL with water, mixed well, put into a bottle and filled with ni...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com