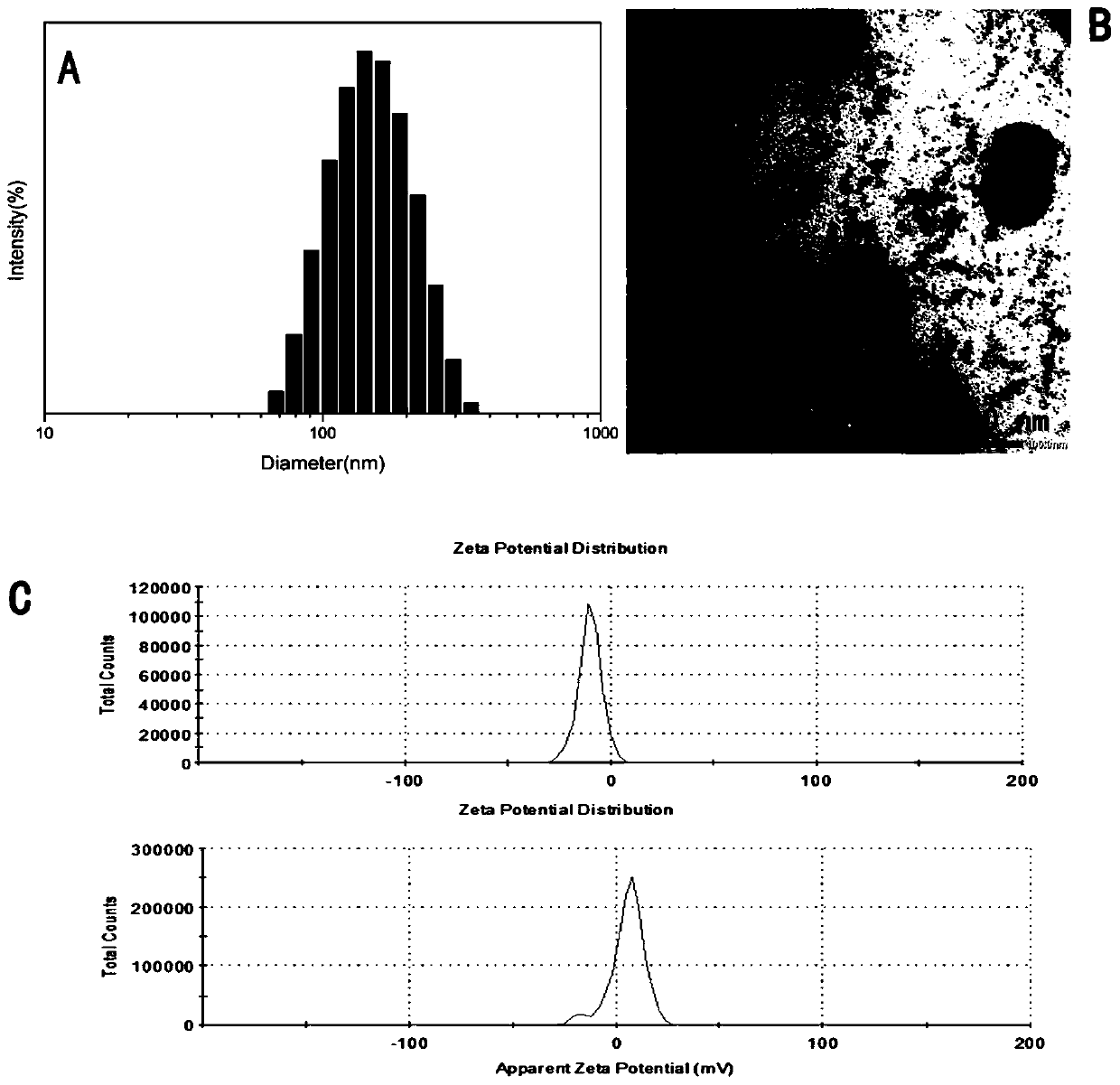
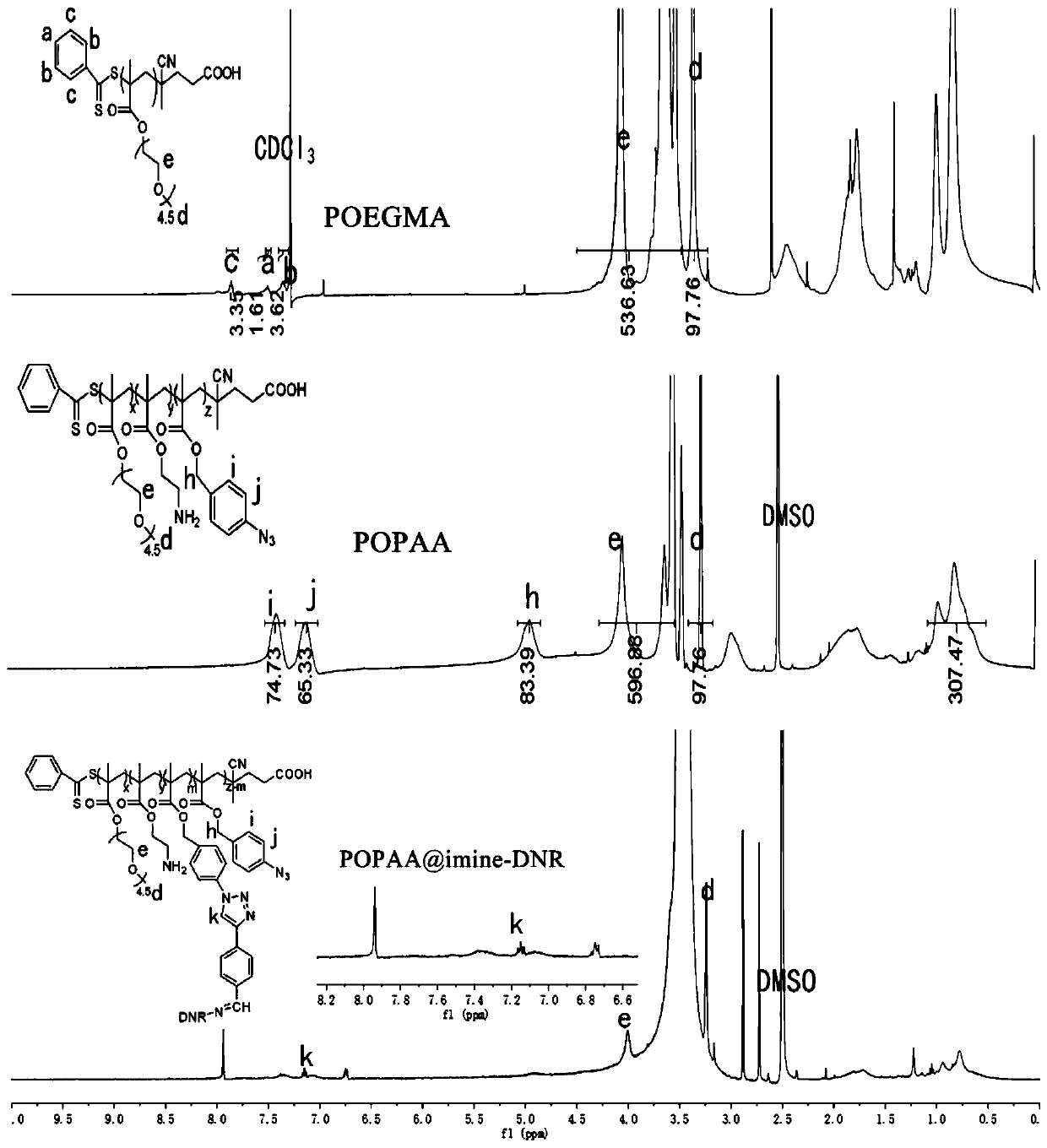
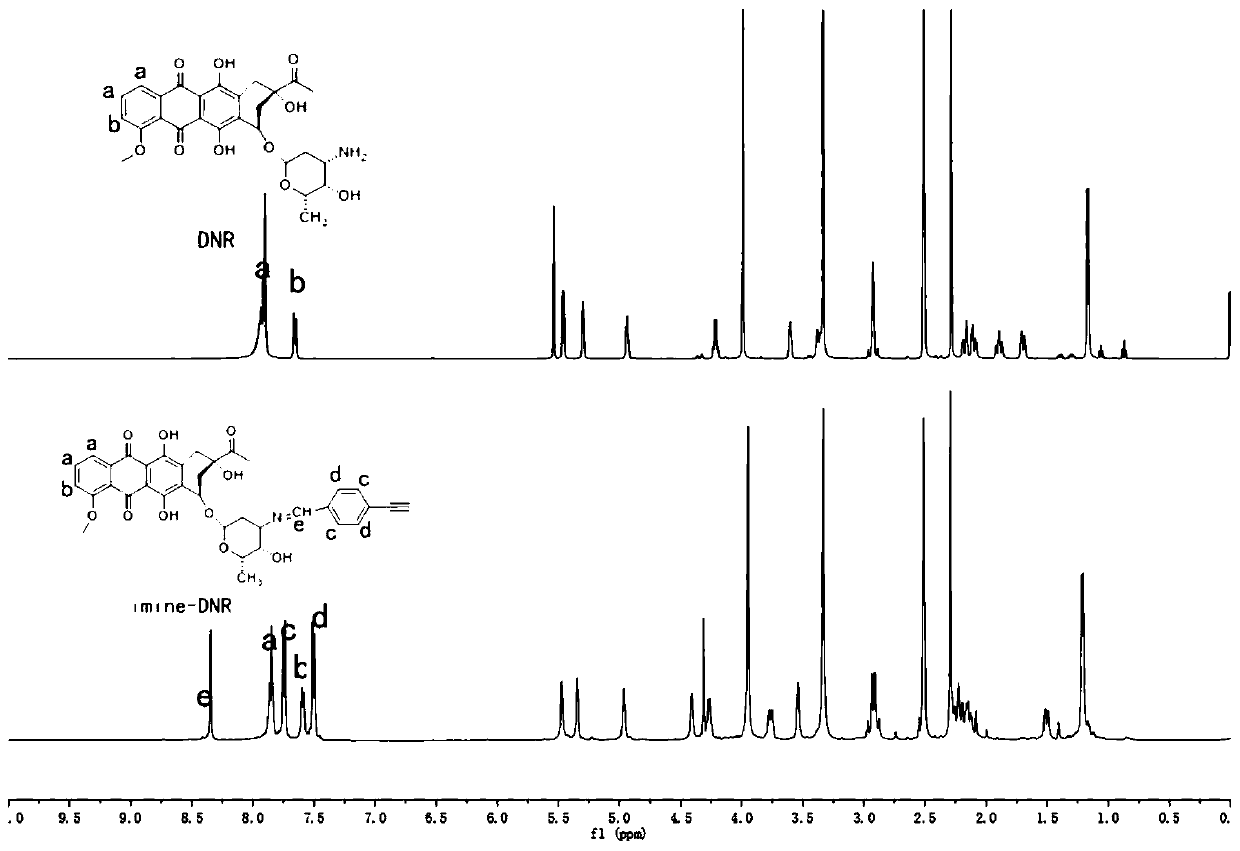
Preparation method of nano delivery system of double-pH sensitive supported chemotherapy drug daunorubicin (DNR)
A daunorubicin and sensitive load technology, applied in the field of nano-delivery system preparation, can solve the problems of reducing the activity of anti-cancer drugs, cardiotoxicity, and damage to cardiac cells, so as to enhance the anti-tumor efficacy, improve targeting, The effect of enhancing intake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A preparation of a nano-delivery system of dual pH-sensitive loaded chemotherapeutic drug daunorubicin, the specific steps are as follows:
[0044] 1) Synthesis of imine bond daunorubicin:
[0045] Get daunorubicin (195mg, 0.035mmol) to be dissolved in the methanol of 20mL, add triethylamine (285 μ L, 2.075mmol), seal, evacuate, vent nitrogen, place in the collector type constant temperature heating magnetic stirrer, at temperature Stir for 2 hours at 30°C to obtain solution A; dissolve p-alkynylbenzaldehyde (54mg, 0.04mmol) in methanol to obtain solution B; add solution B to solution A through a syringe, and continue to react in the dark for 24 hours , rotary evaporated the solvent, added about 1mL of methanol to redissolve, then added about 3mL of acetonitrile to precipitate, centrifuged, discarded the supernatant, and dried in vacuo to obtain the product imine bond daunorubicin imine-DNR.
[0046] 2) Synthesis of polymer carrier:
[0047] Weigh polyethylene glycol ...
Embodiment 2
[0067] 1) the synthesis of imine bond daunorubicin: with embodiment 1
[0068] 2) Synthesis of polymer carrier: same as Example 1
[0069] 3) Synthesis of daunorubicin polymer prodrug intermediate:
[0070] Get POPAA (100mg, 0.0038mmol) after lyophilization, imine-DNR (20mg, 0.03mmol) get sodium ascorbate (1mg, 0.005mmol) and copper sulfate pentahydrate (1.25mg, 0.005mmol), be dissolved in DMF and water The mixed solution (DMF: water = 1: 1) was placed in a 70°C thermostatically heated magnetic stirrer for 24 hours of reaction, dialyzed, and freeze-dried to obtain the daunorubicin polymer prodrug intermediate POPPA@imine-DNR, Wherein the molar ratio of azide in POPAA to imine-DNR is 1:0.2.
[0071] 4) Synthesis of daunorubicin polymer prodrug:
[0072] Take 60 mg of POPPA@imine-DNR obtained in step 3), 5.04 mg of 2,3-dimethylmaleic anhydride, dissolve in DMSO and continue to react for 2 hours, dialyze, freeze-dry to obtain daunorubicin polymer prodrug DA- POPAA@imine-DNR, wh...
Embodiment 3
[0079] 1) Synthesis of imine bond daunorubicin: same as implementation case 1
[0080] 2) Synthesis of polymer carrier: same as implementation case 1
[0081] 3) Synthesis of daunorubicin polymer prodrug intermediate:
[0082] Get POPAA (100mg, 0.0038mmol) after lyophilization, imine-DNR (100mg, 0.15mmol) take sodium ascorbate (3mg, 0.015mmol) and copper sulfate pentahydrate (3.75mg, 0.015mmol), be dissolved in DMF and water The mixed solution (DMF:water=1:1) was placed in a magnetic stirrer with collector type constant temperature heating at 70°C for 24h. Dialysis and freeze-drying can obtain daunorubicin polymer prodrug intermediate POPPA@imine-DNR, wherein the molar ratio of azide in POPAA to imine-DNR is 1:1.
[0083] 4) Synthesis of daunorubicin polymer prodrug:
[0084] Take 60 mg of POPPA@imine-DNR obtained in step 3), 5.04 mg of 2,3-dimethylmaleic anhydride, dissolve in DMSO and continue to react for 2 hours, dialyze, freeze-dry to obtain daunorubicin polymer prodru...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com